Oxazinines
The oxazinins are a group of polycyclic marine toxins that have so far only been found in nature in Mediterranean mussels ( Mytilus galloprovincialis ) and dinoflagellates . All members have a central, morpholine- like ring ( 3-oxo-1,4-oxazinane ), which is connected to an indole as well as a phenol or p -cresol substituent. Since the oxazinines apparently serve as cytotoxic defense substances in the mussels , they are examined for their use as medicinal substances ( antibiotics or cytostatics ).
Occurrence and characteristics
Oxazinines were found in the digestive glands of the mussel Mytilus galloprovincialis (O-1 to O-4) and from 2004 to 2007 in adriatic dinoflagellates (O-5, O-6 and preoxazinin-7). The exact function is unknown; However, they are cell-toxic ( cytotoxic ), the representatives with a nitrile group (O-1, O-4, O-5, O-6) exhibiting the highest cytotoxicity. Oxazinin-1 was able to inhibit the growth of cancer cells of the types WEHI 164 and J774 . As drugs, they could be used to kill microorganisms (antibiotics) and cancer cells.
presentation
A working group at Butler University produced oxazinine-3 by reacting bromoacetic acid bromide with the α-amino alcohol (1) (reduction product of tyrosine ) to form intermediate (2) and subsequent reaction with indole (3) to produce the end product (4, oxazinine-3):
Representative
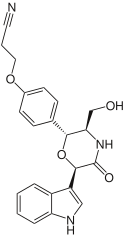
Six representatives of this group of substances have currently been found in nature, as well as a preoxazinin-7 , which may represent a preliminary stage in the biosynthesis from tryptophan and tyrosine:
| Oxazinines | |||||||
| Surname | Oxazinin-1 | Oxazinin-2 | Oxazinin-3 | Oxazinine-4 | Oxazinin-5 | Oxazinine-6 | Preoxazinin-7 |
| CAS | 331836-02-3 | 331836-03-4 | 331836-05-6 | 908290-08-4 | nb | nb | nb |
| structure |  |
 |
 |
 |
 |
 |
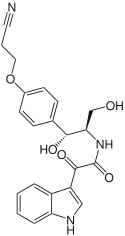
|
swell
Individual evidence
- ↑ a b P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino and S. Magno: toxin from Adriatic blue mussels. A decade of studies. (PDF; 271 kB) In: Pure Appl. Chem. , Vol. 75, No. 2-3, 2003, pp. 325-336.
- ↑ a b L. Grauso: Isolamento e caratterizzazione stereostrutturale di biotossine marine isolate dai mitili contaminati e da dinoflagellati del mar Adriatico. (PDF; 2.5 MB) Dissertation at the Universita Degli Studi di Napoli, 2007.
- ↑ John J. Esteb: Research Interests. (PDF; 154 kB) Department of Chemistry, Butler University .
literature
- JM Richter et al .: Scope and Mechanism of Direct Indole and Pyrrole Couplings Adjacent to Carbonyl Compounds: Total Synthesis of Acremoauxin A and Oxazinin 3 . In: J. Am. Chem. Soc. , 129, No. 42, 2007, pp. 12857-12869, doi : 10.1021 / ja074392m .


