Pechmann reaction
The Pechmann condensation or Pechmann condensation is according to the German chemist Hans Freiherr von Pechmann named (1850-1902) and describes the synthesis of coumarin - derivatives .
Reaction mechanism
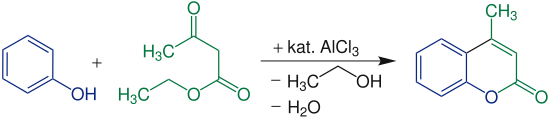
For the synthesis of coumarin derivatives can be β -keto esters with phenols under acidic conditions condense .
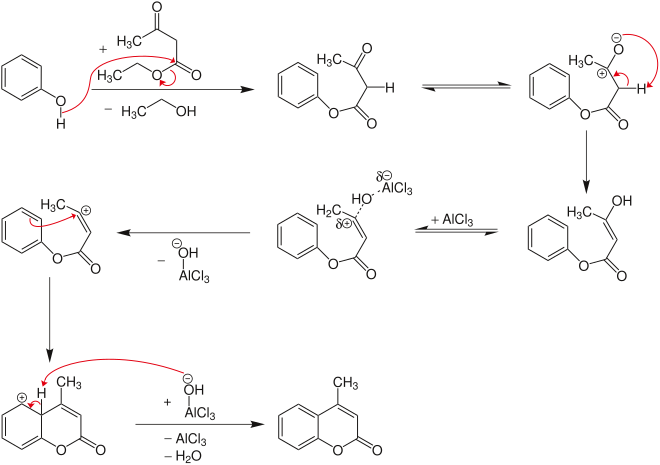
Initially, a transesterification occurs with formation of the phenol ester . This is followed by ring closure , which is similar to Friedel-Crafts alkylation .
Simple phenols have to be used at elevated temperatures in order to obtain a reasonable yield of 80 to 85%.
With more active phenols, such as resorcinol , the reaction can be carried out at room temperature. The yield is then about 85%:
variants
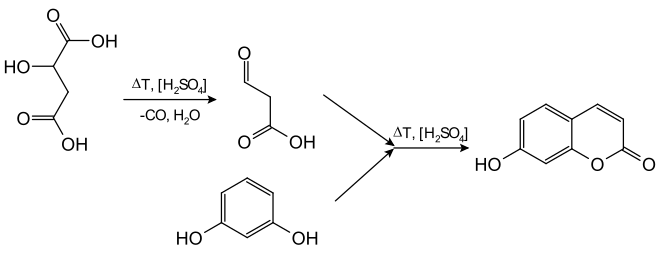
For coumarins that are unsubstituted in the δ-position , the half-aldehyde of malonic acid is required. However, this is unstable and cannot be purchased. This is why it is synthesized in situ from malic acid and sulfuric acid above 100 ° C in order to condense in a Pechmann reaction immediately after its formation. However, the yield is only low.
See also
Individual evidence
- ↑ H. v. Pechmann: New way of forming coumarins. Synthesis of daphnetin . In: Reports of the German Chemical Society . 17, No. 1, 1884, pp. 929-936. doi : 10.1002 / cber.188401701248 .
- ↑ JA Joule, K. Mills Heterocyclic Chemistry , 4th edition, Blackwell Science, Oxford, UK, 2000.
- ^ Eugene H. Woodruff: 4-Methylcoumarin In: Organic Syntheses . 24, 1944, p. 69, doi : 10.15227 / orgsyn.024.0069 ; Coll. Vol. 3, 1955, p. 581 ( PDF ).
source
- Hans Rudolf Christen , Principles of Organic Chemistry, Verlag Sauerländer · Diesterweg · Salle (1977), p 660
- Heterocyclic Chemistry, John A. Joule and Keith Mills, Wiley, p. 239
- March's Advanced Organic Chemistry Reactions, Mechanism and Structure, Michael B. Smith, Jerry March, Wiley, p. 711