Peritectic
A peritectic or peritectic point (derived from the Greek περί = around and τήκω = to melt) is a point in a thermodynamic two-component system that, at constant pressure and with knowledge of the phases involved, is clearly identified by the so-called peritectic temperature (recognizable as a horizontal line in the state diagram) and the peritectic composition is characterized.
In the associated peritectic reaction , a liquid phase S and a solid phase α are in thermodynamic equilibrium with a solid phase β, the chemical composition of which differs from phase α.
Course of the peritectic reaction
If a mixture with any substance fractions of the phases S and α is cooled, the proportion of the mixture which corresponds to the peritectic composition is converted into phase β when the peritectic temperature is reached. Since the composition of phase β does not match either that of phase S or that of phase α, the peritectic reaction is an incongruent phase transition.
If one assumes an idealized, very slow cooling, then the temperature of the mixture only begins to decrease further after the conversion from α to β has been completed. In reality, however, the peritectic transformation usually does not take place completely: The solid phase β is formed at the interface between the phases α and S, so that it can act as a diffusion barrier . As a result, peritectic reactions usually take place much more slowly than the otherwise similar eutectic and eutectoid transformations. Technically relevant periods of time, for example in the production of alloys , are usually not sufficient for complete conversion. The newly created β-mixed crystal is instead deposited around the already existing α-mixed crystal, hence the name peritectic (peritectic, Greek: the built around ).
Examples of peritectic systems
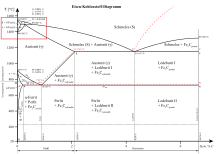
Peritectic transformations usually occur in two-component systems in which the melting points of the components are far apart. An example of a peritectic transformation can be found in the two-component system silver - platinum (Ag – Pt). Platinum has a melting point of 1768 ° C, while silver has a melting point of 982 ° C. Also peritectic is the reaction of the melt and solid δ-iron mixed crystals to solid γ-iron mixed crystals in the iron-carbon system relevant for the casting and production of steel , which occurs at the peritectic temperature of 1493 ° C and the peritectic composition of 0.16 mass% carbon runs off.
Peritectoid reactions
Phase transitions that have the characteristics of peritectic phase transitions, but which take place between solid phases without involving the melt, are referred to as peritectoid .
