Phosphaalkynes
Phosphaalkynes ( English phosphaalkyne ) are chemical compounds having a carbon - phosphorus - triple bond included.
The parent compound, methinophosphide or phosphaethine (HC≡P) is extremely unstable. The first extraction took place through electrical discharges between graphite electrodes in a phosphine atmosphere. The compound was frozen out at −196 ° C and characterized by means of IR spectroscopy (1961 by TE Gier from DuPont). The compound can be stored as a colorless solid at temperatures below −124 ° C. Polymerization occurs at higher temperatures. Phosphate and the resulting fresh polymer are pyrophoric in air . A number of phosphaalkynes have been observed as very short-lived intermediates in gas-phase pyrolysis.
| Short-lived phosphaalkine derivatives (P≡CR) | ||||||||||||
| a | b | c | d | e | f | G | ||||||
| R. | –F | -CH 3 | -CF 3 | -CH = CH 2 | -C≡H | -C≡CC≡N | -C≡N | |||||
| Pyrolysis conditions | 25 ° C / 0.03 torr / KOH | 900 ° C / 0.03 torr | 1000 ° C / 0.03-0.06 Torr | 1000 ° C / 0.03-0.06 Torr | 1100 ° C / 0.03-0.06 Torr | 1100 ° C / 0.08 torr | 700 ° C | |||||
| source | ||||||||||||
In these pyrolyses carried out at high temperatures and low pressures, the starting materials can be corresponding alkylphosphorus dichlorides or trifluoromethylphosphine.
Conjugated phosphaalkynes can be obtained by copyrolysis with phosphorus trichloride .
Finally, phosphaethine can be radically substituted using cyano azide .
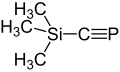
Phenylphosphaethine with a half-life of 7 min at 0 ° C and trimethylsilylphosphaethine with a half-life of 50 min at 20 ° C were found to be somewhat longer-lived compounds . The first phosphaethine tert -butylphosphaethine , which is stable at room temperature , was described in 1981 and its properties were investigated. Adamant-1-ylphosphaethine, which forms colorless crystals with a melting point of 69 - 70 ° C, which can be sublimated without decomposition, is even more stable.
A bis (phosphaalkyne), like the first stable bis (phosphaalken), was produced by Veeck in 1996, but not published. The adamantane framework was used as a sterically demanding framework to avoid both intra- and intermolecular further reactions. Starting from adamantane-1,3-dicarboxylic acid dichloride by reaction with tris (trimethylsilyl) phosphine it was initially possible to obtain adamantane-1,3-bis (phosphaethene) and from this, by elimination of hexamethyldisiloxane, adamantane-1,3-bisphosphaethine could be obtained. However, the bis (phosphaalkyne) was not stable and an intramolecular rearrangement occurred.
In 2003 the first really stable diphosphaalkyne was realized, characterized and analyzed using single crystal diffractometry. This was then also published in the specialist literature. The synthesis of the first stable diphosphaalkyne was achieved through the use of the sterically demanding triptycene framework as the basic structure. This prevented intramolecular reactions from taking place between the two reactive PC groups.
Phosphaalkynes can be used as starting materials for the synthesis of phosphorus-containing heterocycles . Thus cycloaddition form in [3 + 2] with nitrile oxides 1,2,4-Oxazaphosphole 1 with, methyl azide 1,2,3,4-Triazaphosphole 2 , and diazoalkanes 1,2,4-Diazaphosphole 3 .
Individual evidence
- ↑ a b c T. E. Gier: HCP, A Unique Phosphorus Compound . In: Journal of the American Chemical Society , 1961, 83 (7), 1769-1770; doi : 10.1021 / ja01468a058 .
- ↑ a b c Allspach, T .; Regitz, M .; Becker, G .; Becker, W .: A Unusually Coordinated Phosphorus Compounds; 7. Adamant-1-ylmethylidynephosphine, A New, Stable Phosphaalkyne in Synthesis 1986, 31-36, doi : 10.1055 / s-1986-31467 .
- ↑ Regitz, M .; Binger, P .: Phosphaalkynes - Syntheses, Reactions, Coordination Behavior in Angew. Chem. 100 (1988) 1541-1565.
- ↑ Eshtiag-Hosseini, H., Kroto, HW; Brownstein, S .; Morton, JR; Preston, KF: 19 F and 31 P nmr characterization of phospha-alkenes and phospha-alkyne intermediates in the alkaline hydrolysis of trifluoromethylphosphine in J. Chem. Soc. Chem. Commun. 1979, 653-654, doi : 10.1039 / C39790000653 .
- ↑ Hopkinson, MJ, Kroto, HW; Nixon, JF; Simmons, NPC: The detection of the reactive molecule 1-phosphapropyne, CH 3 -C≡P, by microwave spectroscopy in Chem. Phys. Lett. 42 (1976) 460-461, doi : 10.1016 / 0009-2614 (76) 80653-7 .
- ↑ a b c Burckett St. Laurent, JCTR; Cooper, TA; Kroto, HW; Nixon, JF; Ohashi, O .; Ohno, K .: The detection of some new phosphaalkynes, RCP, using microwave spectroscopy in J. Mol. Struct. 79 (1982) 215-220, doi : 10.1016 / 0022-2860 (82) 85054-0 .
- ↑ Durrant, MC; Kroto, HW; McNaughton, D .; Nixon, JF: The new molecule 1-cyano-4-phosphabutadiyne, produced by copyrolysis of PCl3 and CH3-C≡CC≡N: Detection and vibration-rotation analysis by microwave spectroscopy in J. Mol. Spectrosc. 109 (1985) 8-14, doi : 10.1016 / 0022-2852 (85) 90046-3 .
- ↑ Cooper, TA; Kroto, HW; Nixon, JF; Ohashi, O .: Detection of C-cyanophosphaethyne, N≡C – C≡P, by microwave spectroscopy in J. Chem. Soc. Chem. Commum. 1980, 333-334, doi : 10.1039 / C39800000333 .
- ↑ Appel, R. Meier, G.; Reisenauer, HP; Westerhaus, A .: in Angew. Chem. Int. Ed. 20 (1981) 197.
- ↑ Appel, R .; Westerhaus, A .: (CH 3 ) 3 Si - C≡P, a silyl-functional phospha-alkyne in Tetrahedron Lett. 22 (1981) 2159-2160, doi : 10.1016 / S0040-4039 (01) 90486-1 .
- ↑ G. Becker, G. Gresser and W. Uhl: 2.2 dimethylpropylidynephosphine, a stable compound with a phosphorus atom coordination number 1. In: Journal of Nature Research B . 36, 1981, pp. 16-19 ( PDF , free full text).
- ↑ Veeck, W.-G .: 1,2,4-Diazaphosphole with phosphanyl and arsanyl substituents [with an appendix about a bis (phosphaalkyne)] . Dissertation, University of Kaiserslautern, 1997.
- ↑ M. Brym, C. Jones: Synthesis, characterization and reactivity of the first diphosphaalkyne . In: Journal of the Chemical Society , 2003, 3665-3667; doi : 10.1039 / b309061b .