Cyanazide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Cyanazide | ||||||||||||
| Molecular formula | N 3 CN | ||||||||||||
| Brief description |
colorless oil |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 68.04 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| boiling point |
90 ° C |
||||||||||||
| solubility |
soluble in acetonitrile, ethyl acetate, methylene chloride, benzene, toluene, cyclohexane |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cyanazide is a thermally unstable carbon-nitrogen compound with a nitrogen content of 82.35%. It can be assigned to the substance groups of azides and nitriles . It is used in organic synthesis as a reagent for cycloaddition and insertion reactions with alkenes .
Presentation and extraction
Cyanazide can be produced by reacting sodium azide with cyanogen chloride or cyanogen bromide in non-polar solvents such as acetonitrile , ethyl acetate , methylene chloride , toluene or cyclohexane in the temperature range between 10 ° C and 25 ° C.
After the salts have been separated off, the compound can be obtained by distillation. Because of the high instability of the connection, this must be done with extreme caution or avoided if use in solution is possible.
properties
Cyanazide is a colorless oil which can detonate violently under weak thermal or mechanical stress. Handling in solution is relatively safe. The compound is soluble in most organic solvents and in water. In organic solvents it decomposes slowly with the formation of nitrogen . The half-life of a 27% solution in acetonitrile is 15 days at 25 ° C. The solutions in acetonitrile or ethyl acetate can be stored practically undecomposed for a year at temperatures between −20 ° C and 0 ° C. When heated to 40 ° C, slow hydrolysis to carbamazide occurs in water . In 10% sodium hydroxide solution is slowly at room temperature the sodium salt of 5-azido-1 H -tetrazols formed. In a gas phase pyrolysis at 200 ° C, the azodicarbonitrile is formed via the cyanonitrene as an intermediate and nitrogen is split off .
The structure of the compound in the gas phase was investigated using gas-phase electron diffraction measurements . The angled molecule has a bond angle between the azido and cyano groups of 114 °. The cyano group is not completely linearly bound to the azido nitrogen at 175 °.
use
The compound reacts with saturated alkanes with elimination of nitrogen via an intermediate cyanonitrene to form alkyl cyanamides. These can be reduced to the corresponding amines with reducing agents such as lithium aluminum hydride . Investigations on n-hexane , 2,3-dimethylbutane , 2,2-dimethylbutane , cyclohexane , cycloheptane and cyclooctane showed that the reactivity towards CH bonds increases sharply from primary to tertiary structures.
Cyanazid enters into cycloaddition reactions with alkenes, which can lead to aziridines and alkylidenecyanamides. The alkylidenecyanamides are formed via cyclic triazoline intermediates. The product ratio depends on the structure of the alkene. With isobutene as the starting alkene, the products 2-butylene cyanamide and N-cyano-2,2-dimethylaziridine are formed in a ratio of 1.4: 1. With other alkenes, such as with ethene , propene , 1-butene , the two 2-butene isomers , 2-methyl-but-2-ene , 2,3-dimethyl-2-butene , 3,3-dimethyl-1-butene or A similar reaction is observed for 2-hexene with preference for the alkylidenecyanamides as the product.
In the case of cyclopentene, the reaction with cyclic alkenes leads almost completely to the corresponding imine of the cyanamide , which can then be hydrolyzed to cyclopentanone . In contrast, with norbornene, the aziridine is the main product.
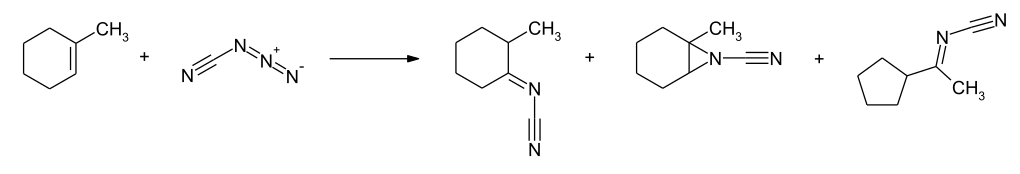
With cycloalkenes with a trisubstituted double bond, a ring reduction can also be observed, as in the example of 1-methylcyclohexene to give the cyclopentane derivative. In the presence of lithium perchlorate , a ring expansion to cycloheptane derivatives can be achieved in the conversion of methylenecyclohexanes .
In reactions at elevated temperature, cyanonitrene is formed as an intermediate from cyanamide with elimination of nitrogen. This reacts with saturated hydrocarbons to form alkyl cyanamides. The ring expansion to cyanoazepine is achieved with benzene . This reaction can also be carried out with toluene , p-xylene , methyl benzoate , chlorobenzene , fluorobenzene, hexafluorobenzene , trifluoromethylbenzene and benzotrichloride with high yield.
By reaction with hydrazine over a Azidohydrazozwischenverbindung which is 1,5-diamino-1 H -tetrazole accessible.
Individual evidence
- ↑ a b c d F. D. Marsh: Cyanogen azide. In: The Journal of Organic Chemistry . 37, No. 19, 1972, pp. 2966-2969, doi: 10.1021 / jo00984a012 .
- ↑ a b c d e f F. D. Marsh, ME Hermes: Cyanogen Azide. In: Journal of the American Chemical Society . 86, No. 20, 1964, pp. 4506-4507, doi: 10.1021 / ja01074a071 .
- ↑ a b c d Encyclopedia of Reagents for Organic Synthesis (e-EROS). John Wiley and Sons, Inc., 1999-2013, entry for Cyanogen Azide, accessed January 24, 2015 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ PG Urben; MJ Pitt: Bretherick's Handbook of Reactive Chemical Hazards . 8th edition, Vol. 1, Butterworth / Heinemann 2017, ISBN 978-0-08-100971-0 , p. 138.
- ^ FD Marsh, ME Hermes: Azodicarbonitrile. In: Journal of the American Chemical Society. 87, No. 8, 1965, pp. 1819-1820, doi: 10.1021 / ja01086a054 , pdf .
- ↑ A. Almenningen, B. Bak, P. Jansen, TG Strand: Molecular structure of gaseous cyanogen azide and azodicarbonitril. In: Acta Chem. Scand. 27, No. 5, 1973, pp. 1531-1540, doi : 10.3891 / acta.chem.scand.27-1531 .
- ↑ a b Anastassiou, AG; Simmons, HE; Marsh, FD: Cyanonitrenes. Reaction with Saturated Hydrocarbons. In: J. Am. Chem. Soc. 87 (1965) 2296-2297, doi: 10.1021 / ja01088a043 .
- ↑ a b c A. G. Anastassiou, HE Simmons: Cyanonitrene. Reaction with Saturated Hydrocarbons. In: J. Am. Chem. Soc. 89 (1967) pp. 3177-3184, doi: 10.1021 / ja00989a018 .
- ↑ a b c d M. E. Hermes, FD Marsh: N-Cyanoaziridines and 1-alkylalkylidenecyanamides from cyanogen azide and olefins. In: The Journal of Organic Chemistry. 37, No. 19, 1972, pp. 2969-2979, doi: 10.1021 / jo00984a013 .
- ↑ John E. McMurry: New ring-enlargement reaction. In: Journal of the American Chemical Society. 91, No. 13, 1969, pp. 3676-3677, doi: 10.1021 / ja01041a059 .
- ↑ John E. McMurry, Anthony P. Coppolino: Cyanogen azide ring expansion reaction. In: The Journal of Organic Chemistry. 38, No. 16, 1973, pp. 2821-2827, doi: 10.1021 / jo00956a019 .
- ^ FD Marsh, HE Simmons: N-Cyanoazepines from Cyanonitrene and Aromatic Compounds. In: Journal of the American Chemical Society. 87, No. 15, 1965, pp. 3529-3530, doi: 10.1021 / ja01093a060 .
- ^ Joo, Y.-H .; Twamley, B .; Garg, S .; Shreeve, JM: Energetic Nitrogen-Rich Derivatives of 1,5-Diaminotetrazole. In: Angew. Chem. 120 (2008) pp. 6332-6335, doi: 10.1002 / anie.200801886 .