Azodicarbonitrile
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
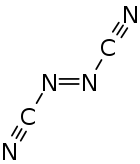 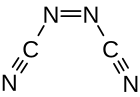
|
|||||||
| trans -form (left) and cis -form (right) | |||||||
| General | |||||||
| Surname | Azodicarbonitrile | ||||||
| other names |
|
||||||
| Molecular formula | C 2 N 4 | ||||||
| Brief description |
orange crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 80.0884 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
|
||||||
| Vapor pressure |
40 mmHg (20 ° C) |
||||||
| solubility |
soluble in benzene, acetonitrile, ethyl acetate, nitromethane |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Azodicarbonitrile is a thermally unstable carbon-nitrogen compound with a nitrogen content of 70%, which can be assigned to the substance groups of azo compounds and nitriles . Azodicarbonitrile occurs in two isomeric forms with the trans and cis compounds .
Presentation and extraction
Azodicarbonitrile can be produced by a gas phase pyrolysis of cyanazide at 200 ° C via the cyanonitrile as an intermediate and nitrogen elimination .
A mixture of isomers is produced during the synthesis.
Another synthesis is based on cyanamide , which is converted into the sodium salt at −50 ° C using sodium methoxide in methanol . An oxidative reaction with tert-butyl hypochlorite at −50 ° C yields the intermediate sodium salt of N -chlorocyanamide . When heated, cyanonitrene is formed in the temperature range between 0 ° C and 10 ° C with elimination of sodium chloride , which dimerizes to form the target compound.
A mixture of isomers is also formed here.
properties
Azodicarbonitrile is a crystalline, orange colored and volatile substance. The melting points observed depend on the manufacturing method. A product made by gas phase pyrolysis melts at 35–37.5 ° C. A product obtained from cyanamide in solution melts at 50-55 ° C. The vapor pressures are around 8 mmHg at 0 ° C, 18 mmHg at 10 ° C and 40 mmHg at 20 ° C. The purification by means of distillation was carried out at temperatures below 0 ° C. and pressures below 1 Torr. Azodicarbonitrile can detonate in closed containers under mechanical stress or when heated. The compound is soluble in benzene , acetonitrile , ethyl acetate , nitromethane and 1,1,2-trichloro-1,2,2-trifluoroethane without decomposition . The solutions in water , methanol and diethyl ether are not stable. The 13 C-NMR spectrum shows only one signal at 118.3 ppm. In the IR spectrum , the CN vibration at 2200 cm −1 and the NN vibration at 1650 cm −1 are significant. Investigations by means of IR and Raman spectroscopy showed that the compound must be in the trans form. With the help of gas-phase electron diffraction measurements it was also found that the trans isomer is essentially present in the gas phase . The bond angle between the azo and cyano groups is 113 °. At 172 °, the cyano group is not completely linearly bound to the azo nitrogen.
Reactions
Azodicarbonitrile reacts smoothly with dienes to form 4,5-diazacyclohexenes. With 2,3-dimethylbutadiene , 1,2-dimethyl-4,5-dicyano-4,5-diazacyclohexene is formed by a [4 + 2] -cyloaddition, with cyclopentadiene 5,6-dicyano-5,6-diazabicyclo is formed [2.2.1] hepten and formed 1,2,3,4-tetrahydro-1,4- o -benzenophthalazine-2,3-dicarbonitrile with anthracene .
Individual evidence
- ↑ a b c d e f g h i j F. D. Marsh, ME Hermes: Azodicarbonitrile. In: Journal of the American Chemical Society . 87, No. 8, 1965, pp. 1819-1820, doi : 10.1021 / ja01086a054 .
- ↑ a b c d e f Mary Gail Kinzer Hutchins, Daniel Swern: Generation of Cyanonitren: Study of the Reaction of Sodium Hydrogen Cyanamide, tert-Butyl Hypochlorite, and Terniary Amines. In: The Journal of Organic Chemistry . 47, No. 25, 1982, pp. 4847-4850, doi : 10.1021 / jo00146a005 .
- ↑ a b c d Børge Bak, Roar Eskildsen, Peter Jansen, Sigfrid Svensson, J. Koskikallio, Sukeji Kachi: Preparation of C 2 N 4 , Azodicarbonitrile. In: Acta Chemica Scandinavica. 25, 1971, pp. 3181-3181, doi : 10.3891 / acta.chem.scand.25-3181 ( pdf ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ PG Urben; MJ Pitt: Bretherick's Handbook of Reactive Chemical Hazards . 8th edition, Vol. 1, Butterworth / Heinemann 2017, ISBN 978-0-08-100971-0 , p. 239.
- ↑ Bak, B .; Jansen, P .: The Symmetry of Azocarbonitrile in J. Mol. Struct. 11 (1972) 25-31, doi : 10.1016 / 0022-2860 (72) 85219-0 .
- ↑ a b Almenningen, A .; Bak, B .; Jansen, P .; Strand, TG: Molecular Structure of Gaseous Cyanogen Azide and Azodicarbonitrile in Acta Chem. Scand. 27 (1973) 1531-1540, doi : 10.3891 / acta.chem.scand.27-1531 , pdf .








