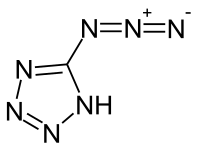
5-azido-1 H tetrazole
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | 5-azido-1 H tetrazole | ||||||
| other names |
|
||||||
| Molecular formula | CHN 7 | ||||||
| Brief description |
colorless crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 111.7 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
1.72 g cm −3 |
||||||
| Melting point |
75 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
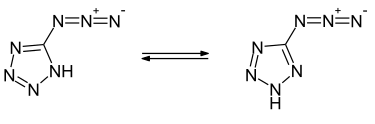
5-Azido-1 H -tetrazole is a heterocyclic, highly explosive chemical compound that belongs to the group of organic tetrazoles and azides . With a nitrogen content of 88.3%, the compound is currently the most nitrogen-rich, synthesized tetrazole derivative.
history
A first synthesis, albeit with poor yield, was described by J. Thiele and H. Ingle as early as 1895. Tetrazylhydrazide was reacted with sodium nitrite and hydrochloric acid. The product obtained was referred to as tetracylazoimide or diazotetrazolimide, the azide structure not being recognized. 5-azido-1 H tetrazole was then described in a patent of W. Friederich and K. Flick again until the 1937th
Extraction and presentation
The synthesis of 5-azido-1 H -tetrazole is carried out by reacting cyanogen bromide with sodium azide , the cyanamide initially being formed as an intermediate, which is further converted to the sodium salt of 5-azido-1 H -tetrazole. The target compound then results from a subsequent treatment with hydrochloric acid .
Another synthesis starts from 5-amino-1 H -tetrazole of which comprises first means of sodium nitrite is converted into the corresponding diazonium compound and hydrochloric acid. The subsequent reaction with sodium azide gives the target compound.
properties
Physical Properties
5-Azido-1 H -tetrazole forms colorless, needle-like crystals, melting at 75 ° C to melt . The compound crystallizes in a monoclinic crystal lattice in the space group P 2 1 / c (space group no. 14) . With an enthalpy of formation of 611 kJ mol −1 , it is a strongly endothermic compound. 5-Azido-2 H -tetrazole can be formulated as the tautomeric isomer . Quantum chemical calculations show that this would be the more stable tautomer in the gas phase. In the crystal lattice or in solution, however, the 1H tautomer is stabilized due to hydrogen bonds.
Chemical properties
The connection is thermally unstable and can decompose explosively. In a DSC measurement, a strongly exothermic decomposition is observed from 165 ° C. It is extremely sensitive to shock and friction . The impact sensitivity is given as <1 J, the friction sensitivity as <5 N.
use
Because of the low stability and high sensitivity to spontaneous explosion, no commercial application could gain acceptance.
literature
- Robert Matyas, Jiri Pachman: Primary Explosives . Springer, 2013, ISBN 978-3-642-28435-9 , pp. 209-212.
Individual evidence
- ↑ a b c d e f g h i j k Jörg Stierstorfer, Thomas M. Klapötke, Anton Hammerl, Robert D. Chapman: 5-Azido-1H-tetrazole - Improved Synthesis, Crystal Structure and Sensitivity Data . In: Journal of Inorganic and General Chemistry . tape 634 , no. 6-7 , 2008, pp. 1051-1057 , doi : 10.1002 / zaac.200800003 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Johannes Thiele, Harry Ingle: About some derivatives of tetrazole . In: Justus Liebig's Annals of Chemistry . tape 287 , no. 3 , 1895, p. 233-265 , doi : 10.1002 / jlac.18952870302 .
- ↑ Patent DE719135 : Process for the production of tetracylazide or its salts. Registered September 14, 1937 , published March 5, 1942 , inventors: W. Friederich, K. Flick.
- ^ FD Marsh: Cyanogenic acid . In: The Journal of Organic Chemistry . tape 37 , no. 19 , 1972, p. 2966-2969 , doi : 10.1021 / jo00984a012 .
- ↑ Anton Hammerl, Thomas M. Klapötke, Peter Mayer, Jan J. Weigand, Gerhard Holl: Synthesis, Structure, Molecular Orbital Calculations and Decomposition Mechanism for Tetrazolylazide CHN 7 , its Phenyl Derivative PhCN 7 and Tetrazolylpentazole CHN 9 . In: Propellants, Explosives, Pyrotechnics . tape 30 , no. 1 , 2005, p. 17-26 , doi : 10.1002 / prep.200400081 .
- ^ Anton Hammerl, Thomas M. Klapötke , Heinrich Nöth, Markus Warchhold, Gerhard Holl: Synthesis, Structure, Molecular Orbital and Valence Bond Calculations for Tetrazole Azide, CHN 7 . In: Propellants, Explosives, Pyrotechnics . tape 28 , no. 4 , 2003, p. 165-173 , doi : 10.1002 / prep.200300001 .
- ^ Anton Hammerl, Thomas M. Klapötke: Tetrazolylpentazoles: Nitrogen-Rich Compounds . In: Inorganic Chemistry . tape 41 , no. 4 , 2002, p. 906-912 , doi : 10.1021 / ic0108134 .