Piperyline
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
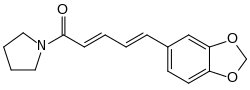
|
||||||||||
| (2 E , 4 E ) shape | ||||||||||
| General | ||||||||||
| Surname | Piperyline | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 16 H 17 NO 3 | |||||||||
| Brief description |
light yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 271.31 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
143-145 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Piperyline is a chemical compound from the benzodioxole group . Despite the similarity of names, it has a different structure from piperylene .
Occurrence
The (2 E , 4 E ) and (2 Z , 4 E ) forms of piperyline occur naturally in black pepper ( Piperis nigri fructus ) and in the leaves of P. trichostachine . The content in black pepper is between 2 to 3 ‰ .
properties
Piperyline fluoresces slightly bluish under UV light at a wavelength of 365 nm.
Individual evidence
- ↑ a b c d data sheet piperylin, analytical standard at Sigma-Aldrich , accessed on October 6, 2018 ( PDF ).
- ↑ a b Shmuel Yannai: Dictionary of Food Compounds with CD-ROM, Second Edition . CRC Press, 2012, ISBN 978-1-4200-8351-4 , pp. 1908 ( limited preview in Google Book Search).
- ↑ Kenji Hirasa, Mitsuo Takemasa: Spice Science and Technology . CRC Press, 1998, ISBN 978-0-585-36755-2 , pp. 45 ( limited preview in Google Book search).
- ^ Shinji Funayama, Geoffrey A. Cordell: Alkaloids A Treasury of Poisons and Medicines . Elsevier, 2014, ISBN 978-0-12-417314-9 , pp. 130 ( limited preview in Google Book search).
- ↑ TK Lim: Edible Medicinal And Non-Medicinal Plants Volume 4, Fruits . Springer Science & Business Media, 2012, ISBN 978-94-007-4053-2 , p. 327 ( limited preview in Google Book search).
- ^ Sabine Bladt, Eva M. Zgainski: Plant Drug Analysis A Thin Layer Chromatography Atlas . Springer Science & Business Media, 2013, ISBN 978-3-662-02398-3 , pp. 248 ( limited preview in Google Book search).
