Plasma genes
| This item has been on the quality assurance side of the editorial chemistry entered. This is done in order to bring the quality of the articles on the subject of chemistry in terms of form and content to the level desired in Wikipedia. We are grateful for your help , please take part in the discussion ( new entry ) or revise the article accordingly. |
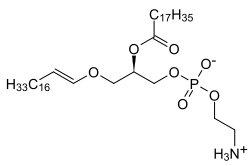
Plasmalogens are ether lipids that are structurally similar to phosphatidylcholines and phosphatidylethanolamines . The difference is that instead of a fatty acid ester, an enol ether is present on the terminal carbon atom of the glycerol . The second fatty acid, bound as an ester , is mostly polyunsaturated. Over 10% of the phospholipids in the brain and muscles belong to the group of plasmalogens. Since there seems to be a connection between plasma genes and various neuropathologies, they are the subject of intensive research.
They were discovered by Robert Feulgen .
Functions
Plasmalogens are found in many human tissues, especially in large amounts in the nervous, immune and cardiovascular systems. Tissue-specific it can be differentiated that in the human heart more plasmalogens with choline as the head group (so-called phosphatidylcholine- plasmalogens) occur, while in the myelin sheaths of the nervous system mainly phosphatidylethanolamines with ethanolamine as the head group can be found.
Although the functions of the plasmalogens are not yet fully understood, it has been shown that they protect mammalian cells against oxidation damage. It is also assumed that they play a role in signal transduction .
biosynthesis
The biosynthesis of the plasmalogens takes place in the peroxisomes and in the endoplasmic reticulum (ER). At the beginning, a complex of the enzymes GNPAT (glycerol phosphate acyltransferase) and AGPS (alkyl glycerol phosphate synthase) is formed on the luminal side of the peroxisomal membrane. It has been shown that if z. B. the AGPS the cooperating enzyme GNPAT suffers a loss of activity. The first step of the synthesis is initiated by GNPAT. It acylates DHAP (dihydroxyacetone phosphate) at the sn-1 position. Then the AGPS exchanges the acyl radical with an alkyl radical. The resulting 1-alkyl-DHAP is reduced to 1-O-alkyl-2-hydroxy-sn-glycerophosphate (GPA). This reaction is catalyzed by an acyl / alkyl DHAP reductase, which is located on the ER and on the peroxisomal membrane.
All further modifications now take place in the ER. First, an acyl residue is attached to the sn-2 position by an alkyl / acyl-GPA acyl transferase, the phosphate group being removed by a phosphatase . This produces 1-O-alkyl-2-acyl-sn-glycerol. A phosphotransferase now forms 1-O-alkyl-2-acyl-sn-GPEtn with CDP-ethanolamine. After dehydration at the 1- and 2-position of the alkyl group by an electron transport system and a plasmenylethanolamine desaturase , the PL-typical vinyl ether bond is finally formed. Plasmenylcholine is produced from 1-O-alkyl-2-acyl-sn-glycerol with the help of choline phosphotransferase.
Since there is no plasmenylcholine desaturase, choline PLs can only arise through a detour. Ethanolamine PLs are hydrolyzed to 1-O- (1Z-alkenyl) -2-acyl-sn-glycerol and modified by the enzyme choline phosphotransferase with CDP-choline so that choline PLs are formed.
Pathologies
The Zellweger syndrome is u. a. characterized by a plasmalogen deficiency, as the peroxisomes contain almost no enzymes and are broken down. So they can no longer synthesize the plasma genes.
Reduced levels of plasmalogens in the brain have been associated with Alzheimer's disease , X-linked adrenoleukodystrophy , and Down's syndrome .
Original quotes
- ↑ P. Brites, HR Waterham, RJ Wanders, Functions and biosynthesis of plasmalogens in health and disease, Biochim. Biophys. Acta 1636 (2004) 219-231.
- ↑ J. Biermann, WW Just, RJ Wanders, H. Van Den Bosch, Alkyl-dihydroxyacetone phosphate synthase and dihydroxyacetone phosphate acyltransferase form a protein complex in peroxisomes, Eur. J. Biochem. 261 (1999) 492-499.
- ↑ D. Hardeman, H. van den Bosch, Topography of ether phospholipid biosynthesis, Biochim. Biophys. Acta 1006 (1989) 1-8.
- ^ AJ Brown, F. Snyder, Alkyldihydroxyacetone-P synthase. Solubilization, partial purification, new assay method, and evidence for a ping-pong mechanism, J. Biol. Chem. 257 (1982) 8835-8839.
- ^ PF James, AC Lake, AK Hajra, LK Larkins, M. Robinson, FG Buchanan, R. A Zoeller, An animal cell mutant with a deficiency in acyl / alkyl-dihydroxyacetone-phosphate reductase activity. Effects on the biosynthesis of ether-linked and diacylglycerolipids, J. Biol. Chem. 272 (1997) 23540-23546.
- ↑ TC Lee, Biosynthesis and possible biological functions of plasmalogens, Biochim. Biophys. Acta 1394 (1998) 129-145.
- ^ NE Braverman, AB Moser, Functions of plasmalogen lipids in health and disease, Biochim. Biophys. Acta (2012), doi : 10.1016 / j.bbadis.2012.05.008 .
- ↑ R. Wanders, H. Waterham: peroxisomal disorders: the single peroxisomal enzyme deficiencies. In: Biochimica et Biophysica Acta (2006) - Molecular Cell Research Volume 1763, p. 1707. doi : 10.1016 / j.bbamcr.2006.08.010 .
- ↑ MOW Grimm, J. Kuchenbecker, TL Rothhaar, S. Grösgen, B. Hundsdörfer, VK Burg, P. Friess, U. Müller, HS Grimm, M. Riemenschneider, T. Hartmann: Plasmalogen synthesis is regulated via alkyl-dihydroxyacetone phosphate synthase by amyloid precursor protein processing and is affected in Alzheimer's disease. In: Journal of Neurochemistry (2011), Vol. 116, No. 5, pp. 916-925. doi : 10.1111 / j.1471-4159.2010.07070.x . PMID 21214572 .
- ↑ M. Khan, J. Singh, I. Singh: Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. In: Journal of Neurochemistry (2008), Volume 106, No. 4, doi : 10.1111 / j.1471-4159.2008.05513.x . PMC 2575097 (free full text). PMID 18540993 .
- ↑ EJ Murphy, MB Schapiro, SI Rapoport, HU Shetty: Phospholipid composition and levels are altered in Down syndrome brain. In: Brain Research (2000), vol. 867, no. 1-2, pp. 9-18. doi : 10.1016 / S0006-8993 (00) 02205-8 . PMID 10837793 .
literature
- Nagan N, Zoeller RA: Plasmalogens: biosynthesis and functions . In: Prog Lipid Res.. . 40, No. 3, May 2001, pp. 199-229. PMID 11275267 .
- de Vet EC, Ijlst L, Oostheim W, et al. : Ether lipid biosynthesis: alkyl-dihydroxyacetonephosphate synthase protein deficiency leads to reduced dihydroxyacetonephosphate acyltransferase activities . In: J. Lipid Res . 40, No. 11, November 1999, pp. 1998-2003. PMID 10553003 .
Web links
- D'Eustachio / reactome: plasmalogen biosynthesis