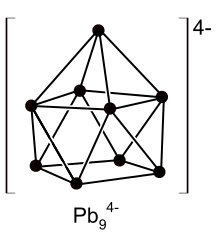
Polyanion
Polyanions are particles made up of several atoms of the same element that have gathered together and, as a group, have a negative charge. They can be obtained by the condensation of several simple anions with the elimination of water. The terms polyanion and polycation were coined by Eduard Zintl around 1930 .
Anion formers are halogens (fluorine, chlorine, bromine, iodine), chalcogens (oxygen, sulfur, selenium and tellurium) and elements further left in the periodic table such as lead , tin , antimony or bismuth . Elements that are more than four main groups before the noble gases in the periodic table (e.g. indium , thallium , mercury ) belong to the non-anion formers. Only insoluble compounds are formed here, which form typical alloy structures. A distinction can therefore be made between: anion formers and non-anion formers, i.e. elements that are one to four main groups before the noble gases and are capable of forming anions or polyanions, and elements further left in the periodic table that do not have this property. This border between the third and fourth main group was referred to in an obituary to Eduard Zintl by Fritz Laves as the Zintl border or Zintl line . It is still known by this name today, even if it is now viewed as less generally valid and less sensible than previously assumed.
In the meantime, it has also been possible to produce indium polyanions - not in liquid ammonia, but in reactions without solvents at much higher temperatures. Such polyanions of the heavier metals, which were previously only known in liquid ammonia, were later named Zintl ions in honor of their discoverer .
In a broader sense, negatively charged polyelectrolytes, e.g. B. in cation exchangers and inorganic polyphosphates , referred to as polyanions.
swell
- IUPAC : Nomenclature of polyanions (PDF; 572 kB)
- Polyanions of the main group elements (PDF; 52 kB)
- Uni-Bonn: Cluster