Eduard Zintl
Eduard Zintl (born January 21, 1898 in Weiden in the Upper Palatinate ; † January 17, 1941 in Darmstadt ) was a German chemist , after whom the chemical substance classes of the Zintl phases and Zintl ions are named, as well as the Zintl limit between 3. and 4th main group in the periodic table of the elements .
Life
School education and studies
Eduard Zintl spent his school days in Weiden and Bayreuth . After moving to Munich with his family , he passed the Abitur exam at the age of 18. Since the First World War was raging in Europe at that time , Zintl was drafted into military service. He could not begin his chemistry studies at the Bavarian Academy of Sciences until the age of 21. But that didn't stop him from pursuing an unprecedented academic career. Already in the first semesters he stood out for his above-average academic performance - Otto Hönigschmid , head of the German Atomic Weight Laboratory, also became aware of him: He made him a Famulus (assistant) and made his private laboratory available to him, where Eduard Zintl worked on determining important atomic weights (Bromine, antimony, silver, gold, ...) involved. He received special permission to begin his dissertation even before he had passed his second association examination.
Doctorate and habilitation
Zintl received his doctorate in 1923 with the thesis "Revision of the atomic weight of bromine through complete synthesis of bromine silver". As a private assistant in the atomic laboratory, he then supervised Otto Hönigschmid's doctoral students (including Günther Rienäcker and Josef Goubeau ) and devoted himself to potentiometric titration , a quantitative analysis method , which had hitherto been neglected . He soon became a luminary in this field .
In addition, he wrote a textbook entitled "Introduction to the Study of Inorganic Chemistry". Inspired by recent studies by Charles August Kraus , the deep blue solutions of metals in liquid ammonia found his ever increasing interest. In Munich he began to research them more closely. Two years after his doctorate , Zintl completed his habilitation in chemistry in 1925.
1928 to 1933: Professor at the University of Freiburg
Zintl remained at the Munich Academy as a curator until he accepted an appointment as an associate professor at the Albert Ludwig University of Freiburg in 1928 . Here he became head of the inorganic department of the chemical laboratory. Little distracted from events outside of his research work, Eduard Zintl developed a topic with which his name remains connected: the field of intermetallic phases (compounds of two or more metals) - more precisely: the Zintl ions and Zintl phases that were later named after him. His first, rather short publication on this subject appeared in 1929: “Salt-like compounds of sodium and their transition to intermetallic phases”. In 1931 more detailed reports of work with lead, tin and many other elements in liquid ammonia followed. In 1938 the Association of German Chemists awarded him the Liebig commemorative coin for investigations into intermetallic phases .
Zintl and his colleagues developed methods to examine air-sensitive substances using X-ray diffractometry . Air-unstable compounds such as the alkali metal hydrides can thus be examined and described in more detail.
In his lectures at the University of Freiburg, Zintl did not convey the peculiarities of the individual chemical substances, but rather gave an overview of the basics that were necessary to better understand the content of the textbooks. Because “he didn't want to read out what was to be read” (quote from an obituary for Eduard Zintl).
Professor at the TH Darmstadt from 1933
The chemist was appointed full professor at the Technical University of Darmstadt in 1933. On October 1 of that year he took over the position of director of the Institute for Inorganic Chemistry as the successor to Lothar Wöhler . As early as 1923 he had stated in his textbook that modern inorganic chemistry was applied physical chemistry; He was now able to make this point of view clear for everyone: Zintl set up a physical-chemical department at the TH Darmstadt and gave the combined institute the name “Institute for Inorganic and Physical Chemistry”.
But it gradually became clear that there was not enough space in this institute, as new chemical apparatus had been developed in the meantime, which had become indispensable for laboratory work, but which took up a lot of space. Zintl organized a spacious, modern new building in the immediate vicinity of the old building. The foundation stone was laid on October 1, 1937. Zintl also modernized the curriculum for chemistry students in Darmstadt. He set up a new course in which basic training was followed by training for advanced students. The Reich Ministry of Education later adopted this concept in a radical reform of the chemistry course at all universities and technical colleges.
In contrast to many university professors who only devoted themselves to research at their institutes, Zintl also cooperated with the chemical industry and became an employee of IG Farbenindustrie AG. From this collaboration, u. a. new research on oxo compounds and oxides emerges. But despite this influence on the part of industry, his research and teaching did not take on the character of functional research : Zintl remained true to basic research . At the Reich Labor Conference of German Chemists in Bayreuth in 1938, he summarized his views on research:
“In science, however, we […] pursue a long-term policy, and we strive for a comprehensive theory through basic research, because it brings us closer to the highest goal of all sciences. It consists in predicting something new. [...] With this, however, all basic research ultimately becomes functional research in the long term. "
In addition, he was in charge of editing the journal for inorganic and general chemistry .
Zintl died on January 17, 1941 of a serious illness and did not live to see him move into the institute he had planned. At his memorial service on January 21, 1942, the new Zintl building was named "Eduard Zintl Institute for Inorganic and Physical Chemistry" in honor of it.
Polyanions and the Zintl Limit
According to reports by A. Joannis in the 1890s, the reaction of lead with sodium in liquid ammonia produced intensely green-colored solutions. Eduard Zintl was interested in the chemistry behind this reaction. He used the methods of potentiometric titration and electrolytic transfer , which he was skilled in, to study this phenomenon. In doing so, he proved for the first time that the colored solutions had a polar character and thus contained charged particles.
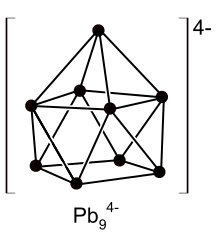
Eduard Zintl came to the conclusion that this type of particle was a polyanion, i.e. several atoms of the same element that had accumulated and had a negative charge as a group. The alkali metal cation, which is coated with solvent molecules, served as the counterion. In the described reaction of lead with sodium in liquid ammonia, a polyanionic salt with the composition [Na (NH 3 ) x ] + 4 [Pb 9 ] 4− was formed . However, the polyanions disintegrated as soon as he completely removed the solvent.
It had long been known that halogens (fluorine, chlorine, bromine, iodine) and chalcogens (oxygen, sulfur, selenium and tellurium) could form anions. But Eduard Zintl had now shown that elements further left in the periodic table (such as lead ) were able to do this. In subsequent investigations, he also demonstrated polyanions of other elements - namely tin , antimony and bismuth .
However, there were elements whose reaction with sodium in liquid ammonia never resulted in polyanions - and these were elements that are more than four main groups before the noble gases in the periodic table (e.g. indium , thallium , mercury ). Here, only insoluble compounds were formed, which formed typical alloy structures. These were the same products that were obtained by melting the pure metals together.
Zintl differentiated between anion formers and non-anion formers, i.e. elements that are one to four main groups before the noble gases and are capable of forming anions or polyanions, and elements further left in the periodic table that do not have this property. This border between the third and fourth main group was referred to in an obituary to Eduard Zintl by Fritz Laves as the Zintl border or Zintl line . It is still known by this name today, even if it is now viewed as less generally valid and less sensible than previously assumed. (Because in the meantime it has also been possible to produce polyanions of indium - not in liquid ammonia, but in reactions without solvents at much higher temperatures.)
Such polyanions of the heavier metals, which were previously only known in liquid ammonia, were later named Zintl ions in honor of their discoverer .
Intermetallic phases
Eduard Zintl did not find any polyanions in the reaction of elements of the 3rd main group (e.g. thallium ) with sodium in liquid ammonia. Instead, it was given an alloy-like system. In this intermetallic compound (i.e. the connection between the two metals sodium and thallium) he demonstrated a new, as yet unknown structure: the NaTl structure . What is special is that the atoms of the two elements (thallium and sodium) are arranged independently of one another in the same way as the carbon atoms in diamond .
For the first time he recognized the principle behind the structure of many intermetallic phases: the anions form a structure that corresponds to that which an element with the same number of valence electrons also occupies. In this case, the formally simply negatively charged thallium ion (with four valence electrons) forms a structure that carbon (in the fourth main group, i.e. also with four valence electrons!) Forms the same due to the same bond . This law is known today as the classic Zintl concept , it was later expanded by Wilhelm Klemm and E. Busmann.
Inspired by these new results, Eduard Zintl systematically investigated many other connections between metals. Fritz Laves then introduced the term Zintl phases in the aforementioned obituary for Eduard Zintl . He had so successfully come up with an umbrella term for the many intermetallic compounds that Zintl had described.
Nowadays the term Zintl phase means intermetallic phases with strongly ionic bond components, i.e. substances in which the type of bond represents a transition form between metal and ionic bond. These are the alkali and alkaline earth compounds with metals or semimetals of the third to fifth main group, i.e. of components with a relatively high difference in electronegativity . The prototype of the Zintl phases is still NaTl . Since Zintl phases have an ionic structure, their enthalpies of formation are comparable to those of typical salts due to the additional lattice energies. They have salt-like brittleness and melting points that are higher than those of the metal components. In addition, unlike alloys, they dissolve well in coordinating solvents such as liquid ammonia.
literature
- HW Kohlschütter: Natural Sciences. 17, 1941, pp. 240-244. doi: 10.1007 / BF01479156
- F. Laves: Natural Sciences. 17, 1941, pp. 244-255. doi: 10.1007 / BF01479157
- Uta Deichmann: Escape, join in, forget. Chemists and biochemists during the Nazi era. Wiley-VCH, Weinheim 2001, ISBN 3-527-30264-6 .
Web links
Individual evidence
- ↑ A. Joannis: Action of sodammonium and potassammonium on metals. In: CR Hebd Seances Acad Sci. 113, 1891, pp. 795-798.
| personal data | |
|---|---|
| SURNAME | Zintl, Eduard |
| BRIEF DESCRIPTION | German chemist |
| DATE OF BIRTH | January 21, 1898 |
| PLACE OF BIRTH | Pastures in the Upper Palatinate , Upper Palatinate |
| DATE OF DEATH | January 17, 1941 |
| Place of death | Darmstadt |