Polycation
Polycations are particles made up of several atoms of the same element that have gathered together and, as a group, have a positive charge. The number of known polycationic compounds of main group elements is far fewer than that of polyanionic compounds. Examples are the cations of the chalcogens such as S 4 2+ , S 8 2+ , Se 10 2+ or Te 6 2+ , which are formed when the elements react with Lewis acids under oxidizing conditions . The terms polyanion and polycation were coined by Eduard Zintl around 1930 .
presentation
Oxidizing agents such as B. Peroxodisulfuryldifluorid S 2 O 6 F 2 in fluorosulfuric acid HSO 3 F or sulfur trioxide SO 3 in sulfuric acid lead selenium (Se) or tellurium (Te) into cations. In addition, the cations are formed by comproportionation or disproportionation of Se, Te in AlCl 3 -containing SO 2 solutions. The tendency of selenium and tellurium to form cations is overall greater than that of the more electronegative sulfur , and the acidity of the Se and Te cations is smaller than that of the sulfur cations.
The red Te 4 2+ - cation ( Tetratellur dication ), for example from elemental tellurium by oxidation in hot concentrated sulfuric acid (H 2 SO 4 are shown). Part of the sulfuric acid is reduced to sulfuric acid (H 2 SO 3 ) during the reaction , which, due to the high temperatures, breaks down into water (H 2 O) and its anhydride sulfur dioxide (SO 2 ), which escapes as a gas:
This reaction serves as a qualitative proof of tellurium in an analysis mixture.
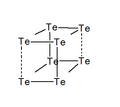
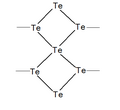
Structures
Structures of different polycations of the chalcogens:
swell
- Ulrich Müller: Inorganic Structural Chemistry . Vieweg + Teubner, Wiesbaden 2008, ISBN 978-3-8348-0626-0 , p. 204 f .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 622.
- University of Giessen: Investigations on the reactivity of chalcogen polycations hexachlorozirconates and hafnates (PDF; 3.7 MB)
- Uni-Bonn: Cluster
- Uni-Bonn: Gallery