Polyureas
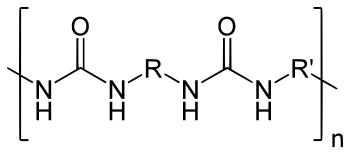
Polyureas (in German is also Polyurea use) are polymers represented by the polyaddition of isocyanates and amines are formed. The polymer has a structural element that is similar to urea : - [ -NH -R- NH- (C = O) -NH -R'- NH- (C = O) - ] n - Structurally, they belong to the aminoplasts .
Manufacturing
The isocyanates used can be monomeric (with at least 2 isocyanate groups), polymeric or so-called prepolymers are used, which are prepared from polyols with an excess of isocyanates and therefore contain isocyanate end groups.
The required amines can be added as a second component or else can be generated from the isocyanates by reaction with water.
Here, too, you have two options:
- the water is added as the second component (two-component systems, usually in combination with polyols) - this is a common method for the production of polyurethane foams
- curing takes place with steam from the air (one-component systems)
Both processes produce carbon dioxide , which causes the polymer to foam. However, one-component systems can be used to produce homogeneous, non-foamed coatings if the layers are thin enough so that the carbon dioxide can diffuse out of the polymer layer quickly enough . This is used with one-component polyurethane paints. If you want to achieve thicker layers, the CO 2 z. B. be bound with the help of calcium oxide . Another possibility of producing non-foaming one-component coatings is to combine so-called latent hardeners with isocyanates. This can e.g. B. oxazolidines , which hydrolyze with water to form amino alcohols , which then react with the isocyanate groups to form urea and urethane groups.
properties
Two-component systems made of aliphatic amines and isocyanates usually react very quickly due to the high nucleophilicity of the amines and must therefore be processed mechanically with two-component mixing systems - the pot lives are in the range of seconds. However, there are also significantly less reactive, mostly aromatic amines available that can be processed by hand. These include B. 4,4'-methylene-bis (2-chloroaniline) (MBOCA), bis-Methylthiotoluylendiamin, N , N ' -bis ( sec butylamino) diphenylmethane and diethyltoluylenediamine. In addition, aliphatic amines are also available, such as polyaspartic acid esters , which also react relatively slowly but, unlike the aromatic amines, do not tend to discolour. Polyureas have good to very good chemical resistance as well as high elasticity and tear resistance.
use
swell
- Kittel, Textbook of paints and coatings, 2nd edition, Volume 2: Binder for solvent-based and solvent-free systems, Ed. Walter Krauß, S. Hirzel Verlag 1998, ISBN 3-7776-0886-6 .
- Entry to polyureas. In: Römpp Online . Georg Thieme Verlag, accessed on June 18, 2014.
Web links
- Patent US4426488 : Elastomeric composition. Published January 17, 1984 , inventor: RANSOME J WYMAN.