Polyhydantoins
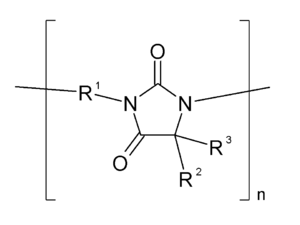
Polyhydantoine refers to a group of polymers or plastics that structurally belong to the imidazolidinediones .
application
Polyhydantoins with dimethyl- substituted rings and with R 1 as the aromatic building block are particularly thermally stable and are therefore used in heat-resistant electrical insulating varnishes, such as wire varnishes and insulating foils. Long-term use temperatures over 160 ° C and higher are common, heat resistance over 270 ° C and good electrical insulation characterize these products. It is used in liquid form from a solution. The polymer itself is practically infusible.
Manufacturing
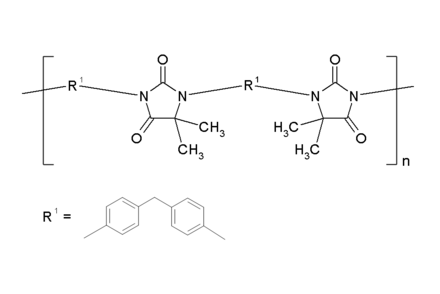
Technically, aromatic diamines are reacted with dimethylchloroacetate , in the second step they are polycondensed with diisocyanates in cresol or toluene as solvents. The polyhydantoin is produced by ring condensation with elimination of the alcohol from the ester groups . Such polymers have a head-to-head arrangement:
Alternatively, polyhydantoins are formed from polyadducts of polyaspartic acid esters and diisocyanates with subsequent heat treatment . Solutions of polyhydantoin in cresol as electrical insulating lacquer raw materials have become known under the name Resistherm ® PH (Bayer AG), but the latter are no longer on the market today.
literature
- Entry on polyhydantoins. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- Rudolf Merten: The synthesis of heterocyclic ring systems for heat-resistant plastics from polyisocyanates. Angewandte Chemie 83 (10), pp. 339-347, 1971.