Prodigiosin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
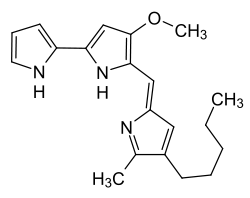
|
||||||||||
| General | ||||||||||
| Surname | Prodigiosin | |||||||||
| other names |
4-Methoxy-5 - [( Z ) - (5-methyl-4-pentyl-2 H -pyrrol-2-ylidene) methyl] -1 H , 1 ' H -2,2'-bipyrrole |
|||||||||
| Molecular formula | C 20 H 25 N 3 O | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 323.43 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Prodigiosin is a metabolic product ( secondary metabolite ) of certain bacteria that has a characteristic red color. The name of this substance, known since the early 20th century, is derived from the English “prodigious” (wonderful).
Occurrence
Prodigiosin was first detected in the enterobacterium Serratia marcescens . Furthermore, prodigiosin and structurally similar substances ( derivatives ) have been detected in the marine bacteria Alteromonas rubra and Vibrio gazogenes and a few other types of bacteria. In these bacteria, the molecule can take on passive transmembrane transport of chloride ions .
biosynthesis
Chemically, prodigiosin is a tri- pyrroles . The basic building blocks of biosynthesis are amino acids and acetate , which means that compared to the biosynthesis of porphyrin , succinyl-CoA is replaced by acetyl-CoA . However, even after decades of research, the biosynthesis of this molecule is still not fully understood.
The biosynthesis does not take place under all growth conditions of the bacterium. CAMP is involved in the regulation of the synthesis . When the pH value of the growth medium falls, the color changes from red to green.
Effects
The most striking property of Prodigiosin is its red color. Therefore, it has been proposed to use the biologically produced pigment for coloring fibers.
The substance has shown effects against microorganisms and tumor cells as well as an immunosuppressive agent and has therefore aroused lively research interest.
The colonies of prodigiosin-producing bacteria look like drops of blood and can appear on rotten bread, for example. Believing in blood miracles or wonders of the host , Catholics established the Feast of Corpus Christi in the Middle Ages .
Structurally related substances
Obatoclax , which is structurally related to Prodigiosin , inhibits the Bcl-2 protein and is being developed for the treatment of cancer.
literature
- Khanafari A, Mazaheri M, Fakhr A and FA: Review of Prodigiosin, Pigmentation in Serratia marcescens . (PDF) In: OnLine Journal of Biological Sciences . 6, No. 1, 2006, pp. 1-13.
Individual evidence
- ↑ Data sheet Prodigiosin hydrochloride from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ A b Maurice Moss: Bacterial pigments. (PDF; 164 kB) In: Microbiologist. The Society for Applied Microbiology, 2002, accessed October 13, 2010 .
- ↑ Seganish JL, Davis JT: Prodigiosin is a chloride carrier that can function as an anion exchanger . (PDF) In: Chem. Commun. (Camb.) . No. 46, December 2005, pp. 5781-3. doi : 10.1039 / b511847f . PMID 16307144 .
- ↑ Williams RP: Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens . In: Appl Microbiol . 25, No. 3, March 1973, pp. 396-402. PMID 4572893 . PMC 380817 (free full text).
- ↑ Kwon SK, Park YK, Kim JF: Genome-wide screening and identification of factors affecting the biosynthesis of prodigiosin by Hahella chejuensis, using Escherichia coli as a surrogate host . In: Appl. Environ. Microbiol. . 76, No. 5, March 2010, pp. 1661-8. doi : 10.1128 / AEM.01468-09 . PMID 20038694 . PMC 2832371 (free full text).
- ↑ Kalivoda EJ, Stella NA, Aston MA, et al. : Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens . In: Res. Microbiol. . 161, No. 2, March 2010, pp. 158-67. doi : 10.1016 / j.resmic.2009.12.004 . PMID 20045458 .
- ↑ Sundaramoorthy N, Yogesh P, Dhandapani R: Production of prodigiosin from Serratia marcescens isolated from soil . (PDF) In: Ind. J Sci. Techn. . 2, No. 10, October 2009, pp. 32-34.
- ↑ Alihosseini F, Lango J, Ju KS, Hammock BD, Sun G: Mutation of bacterium Vibrio gazogenes for selective preparation of colorants . In: Biotechnol. Prog . 26, No. 2, March 2010, pp. 352-60. doi : 10.1002 / btpr.346 . PMID 19902486 . PMC 2864118 (free full text).
- ↑ Pandey R, Chander R, Sainis KB: Prodigiosins as anti cancer agents: living up to their name . In: Curr. Pharm. Des. . 15, No. 7, 2009, pp. 732-41. PMID 19275639 .
- ^ A b Pérez-Tomás R, Viñas M: New insights on the antitumoral properties of prodiginines . In: Curr. Med. Chem . 17, No. 21, 2010, pp. 2222-31. PMID 20459382 .
- ↑ Fürster A: Chemistry and Biology of Roseophilin and the Prodigiosin alkaloids: 2500 Years . In: Angew. Chem. . 115, 2003, pp. 3706-3728. doi : 10.1002 / anie.200300582 .
- ↑ geminx.com: GX15-070 (Obatoclax) ( memento February 26, 2009 in the Internet Archive ), accessed October 13, 2010.