Diketopyrrolopyrrole pigments

Diketopyrrolopyrrole pigments ( DPP pigments ) are very solvent and weather-resistant polycyclic colorants that are used in the production of various paints and in the coloring of plastics . Although there are representatives with dyestuff properties, the term DPP mostly refers to the pigments .
history
DPP pigments were first synthesized in 1974 in small quantities using a Reformatzki reaction in the laboratories of what was then Ciba-Geigy (CH-Basel). After extensive work on optimizing the synthesis, the first product came onto the market in 1986. After the patent expired, several competing products, especially for the most important representative, CI Pigment Red 254 , were launched by European and Asian companies from 2004 onwards , which caused a sharp drop in prices.
Chemical structure
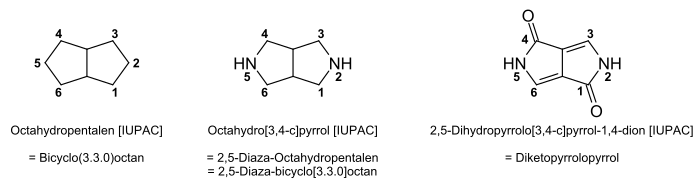
DPP pigments are based on the nitrogen-containing , heterocyclic compound diketopyrrolopyrrole . Two five-membered rings of the substance pyrrole are fused into it ; on two carbon atoms α to the nitrogen atom (positions one and four) the pigments each have a carbonyl group ( diketo ) and are therefore also lactams .
The bicyclic chromophores are formally derived from octahydropentalen ( bicyclo [3.3.0] octane ), in which C-2 and C-5 are replaced by nitrogen atoms ( 2,5-diaza-octahydropentalen or 2.5-diaza-bicyclo [3.3. 0] octane ). The basic body thus obtained is 2,5-dihydropyrrolo [3,4- c ] pyrrole-1,4-dione (according to IUPAC ) or simply diketopyrrolopyrrole .
In the case of the representatives used, the base body is substituted in the 3- and 6-positions with aryl radicals. Newer DPP pigments are also N-substituted, which means that a wider range of colors and modified solution properties can be achieved.
The further substitution of the aryl radicals essentially determines the properties of the most important representatives. The variant with unsubstituted aryl residues is listed in the Color Index under CI Pigment Red 255 .
Manufacturing
Succinic diesters are condensed with double molar amounts of suitably substituted benzonitriles with strong bases. The crude pigment obtained is conditioned by heating in a solvent ( thermal aftertreatment ). The structure can be traced back to the succinic acid through a formal hydrolysis of the base body :
N-substituted aryldiketopyrrolopyrroles can be represented by the condensation of lactones with arylamines in the presence of auxiliaries such as dicyclohexylcarbodiimide ( DCC ) as bright red pigments which have a red to orange-red solid fluorescence.
Main representatives
By far the most important representative of this pigment group is CI Pigment Red 254 , also known colloquially as pyrrole red or DPP red , which differs from CI Pigment Red 255 in that it has a Cl atom in the para position on both aryl groups. A phenyl or tert- butyl group leads to CI Pigment Red 264 (DPP ruby) or CI Pigment Orange 73 (DPP orange). The last three representatives mentioned can be found on the market, but in contrast to the more universally used DPP red, they are used more for specific applications.
properties
DPP pigments produce extremely pure color tones ranging from yellowish orange to bluish violet or ruby (color tone) . In detail, the most important representatives cover the following color ranges:
- CI Pigment Orange 73 → Pure, reddish orange
- CI Pigment Red 255 → Pure, yellowish red or scarlet
- CI Pigment Red 254 → Medium red
- CI Pigment Red 264 → Deep Ruby (shade)
DPP pigments are very resistant to light, weather, solvents, heat and chemicals, making them suitable for high-quality formulations.
Soluble DPPs can also be produced by suitable substituents, which can be used in dye lasers or fluorescence solar collectors because of their large Stokes shift and very good fluorescence quantum yield .
Applications
The most popular application in the world is automotive paint. This fame is essentially based on the distinctive color Ferrari red , the formulations of which are largely based on CI Pigment Red 254 . DPP pigments are also used in the field of high-quality industrial coatings, but here mainly in the high-quality segment. For indoor applications, cheaper azo pigments from the Naphthol AS group are usually used, such as B. CI Pigment Red 112 or CI Pigment Red 170 . In the automotive sector, in addition to CI Pigment Red 254, CI Pigment Red 264 in particular is of importance because, due to its greater transparency, it can also be used for dark red metallic shades.
Due to their high heat and weather resistance , DPP pigments are also used in areas of application that place high demands on temperature resistance . These include, for example, the plastic mass coloring , powder coatings and coil coating (strip coating) used. There, too, the pigments are mainly preferred for outdoor use, while cheaper pigments are used indoors.
Another area of application are emulsion paints . It is also used here mostly outdoors. Since facade paints are far less able to protect the pigments they contain, the use of organic pigments in such a matrix is generally questionable, something even the manufacturers disagree on. Nevertheless, the use of DPP pigments, especially CI Pigment Red 254 and CI Pigment Orange 73, is common.
toxicology
Representatives such as CI Pigment Red 254 (CAS number: 84632-65-5) or CI Pigment Orange 71 (CAS number: 84632-50-5) have such a low toxicity ( LD 50 > 5,000 mg / kg orally or> 2,000 mg / kg dermal) that they may be added to plastics up to 1% as a colorant.
Individual evidence
- ↑ Clariant product brochure
- ↑ CINIC product brochure.
- ↑ a b H. Langhals, T. Grundei, T. Potrawa, K. Polborn: Highly photostable organic fluorescent pigments - a simple synthesis of N-arylpyrrolopyrrolediones (DPP) , In: Liebigs Ann. 1996, 679-682.
- ↑ W. Herbst, K. Hunger: Industrial organic pigments. Wiley-VCH 2004
- ↑ A. Goldschmidt, H.-J. Streitberger: BASF Handbook Painting Technology. Vincentz Network 2002, ISBN 3-87870-324-4 .
- ↑ W. Herbst, K. Hunger: Industrial organic pigments. Wiley-VCH 2004.
- ↑ H. Langhals, T. Potrawa, H. Nöth, G. Linti: The influence of packing effects on the solid fluorescence of diketopyrrolopyrroles (PDF; 1.9 MB), Angew. Chem. 1989, 101, 497-499; Angew. Chem. Int. Ed. Engl. 1989, 28, 478-480.
- ^ Clariant, PA Division; Pigments for architectural coatings; 2007.
- ↑ Ciba; Pigments for decorative coatings; 2007.
- ^ Verband der Mineralfarbenindustrie eV: Colorants for food contact articles and packaging made of plastics. Aspects of product safety (PDF; 1.2 MB), 2002.