RAFT polymerization
The so-called RAFT or reversible addition-fragmentation chain transfer ('Reversible addition-fragmentation chain transfer') - polymerisation is a special form of controlled radical polymerisation . RAFT was first described in 1998 by the working group around Ezio Rizzardo ( CSIRO ) ( Graeme Moad , San Thang , among others ) were involved, based on work by Sam Zard (Paris).
General
First and foremost, it is an independent method for the targeted synthesis of polymers with a well-defined molar mass or degree of polymerization , low polydispersity and known end functionality. Since RAFT is a controlled process, complex molecular architectures such as block, star or comb polymers are also accessible. Control of the reaction is achieved by reversible chain transfer reactions . A growing radical chain adds to the so-called RAFT-agent, creating an intermediate radical. Due to the structure of the RAFT agents, this intermediate has the possibility of fragmenting towards different sides, which in turn regresses a RAFT agent and a radical available for propagation (which, however, expressly does not have to correspond to the original radical chain). In this way, the probability of propagation is evenly distributed over all chains. The average chain length of the polymer formed is proportional to the RAFT agent concentration and the reaction rate. Typical substance classes for RAFT agents are dithioesters , dithiocarbamates , trithiocarbonates and xanthates .
In addition to the so-called Atom Transfer Radical Polymerization (ATRP) and nitroxide-mediated polymerization (NMP), RAFT is one of the best-known and most important representatives of controlled radical polymerization. RAFT owes its widespread use to its compatibility with a large number of monomers and the relatively mild conditions under which the reactions can be carried out. In contrast to ATRP and NMP, RAFT polymerization (albeit with prominent exceptions) does not cause any retardation of the reaction rate, which allows faster and therefore more cost-effective syntheses.
Reaction course
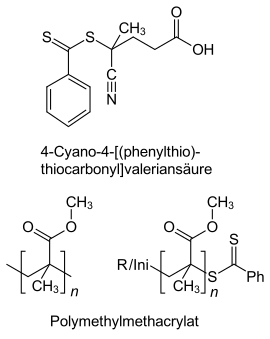
The initiation and a first chain growth (1) takes place in RAFT polymerization with conventional initiators such as AIBN or BPO . Due to the presence of dithiobenzoates, the actively growing chain P n • forms an adduct radical, whereby an equilibrium is established with elimination of R • (2). R • is again an actively growing radical and forms P m • (3). P m • also tends to attach to a dithiobenzoate and equilibrium (4) is established, which is an equilibrium between a "sleeping" and two potentially active states. The actual growth steps correspond to conventional free-radical polymerization , with termination reactions inevitably also occurring (5). (6) shows a common dithiobenzoate and a possible product such as polymethyl methacrylate by polymerization of methyl methacrylate . The main product with the end groups is shown at the bottom right. The dithiocarbonyl group can be reactivated for polymerization and allows the production of AB block copolymers .
literature
- J. Chiefari, YK Chong, F. Ercole, J. Krstina, J. Jeffery, TPT Le, RTA Mayadunne, GF Meijs, CL Moad, G. Moad, E. Rizzardo, SH Thang, Macromolecules 1998 , 31 (16) , 5559-5562. doi : 10.1021 / ma9804951
- "RAFTing Down Under: Tales of Missing Radicals, Fancy Architectures and Mysterious Holes" Barner-Kowollik, C .; Davis, TP; Heuts, JPA; Stenzel, MH; Vana, P .; Whittaker, MJ Polym. Sci. Polym. Chem. 2003, 41, 365-375.
- "Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization, 1: The Current Situation" Barner-Kowollik, C .; Buback, M .; Charleux, B .; Coote, ML; Dragon, M .; Fukuda, T .; Goto, A .; Klumpermann, B .; Lowe, AB; McLeary, J .; Moad, G .; Monteiro, MJ; Sanderson, RD; Tonge, MP; Vana, PJ Poly. Sci. - Polym. Chem. 2006, 44, 5809-5831.
- "Theoretical Description of Reversible Addition Fragmentation Chain Transfer (RAFT) Polymerizations: Conditions for Inhibition, Retardation and Optimum Living Polymerization" Vana, P .; Davis, TP; Barner-Kowollik, C. Macromol. Theory Simul. 2002, 11, 823-835.
Web links
- Dissertation on polymerizations in the presence of RAFT reagents by JAM de Brouwer. Contains u. a. Simulations of the kinetics of the same. (PDF file; 7.32 MB)
Individual evidence
- ↑ Bernd Tieke, Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, pp. 84f.
