Rhenocene
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
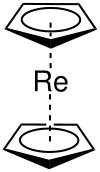
|
||||||||||
| Ecliptic conformation | ||||||||||
| General | ||||||||||
| Surname | Rhenocene | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 10 Re | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 316.39 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Rhenocene (named after IUPAC : bis (η 5 -cyclopentadienyl) rhenium (II) ) with the semi- structural formula [Re (C 5 H 5 ) 2 ] or also [Re (Cp) 2 ] , is a chemical compound of the rhenium group the metallocenes .
Extraction and presentation
Rhenocene can be produced, isolated and investigated by photolysis of Re (C 5 H 5 ) 2 H in a nitrogen and argon matrix at 12 K. A pentamethyl derivative and rhenocene hydride can be isolated above this temperature.
properties
At very low temperatures, the rhenocene complex is monomeric and has a sandwich structure .
use
Rhenocene complexes are examined for their antitumor activity.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on sandwich compounds . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S05468 .
- ↑ a b Jeremy N. Hill, Robin N. Perutz, A. Denise Rooney: Laser-Induced Fluorescence of Rhenocene in Low-Temperature Matrixes: Selective Excitation and Emission . In: J. Phys. Chem. Band 99 , no. 2 , 1995, p. 531-537 , doi : 10.1021 / j100002a014 .
- ↑ subgroup elements, lanthanides, actinides, transactinides Volume 2: transition elements, lanthanides, actinides, transactinide attachments . Walter de Gruyter GmbH & Co KG, 2016, ISBN 978-3-11-049590-4 , p. 1929 ( limited preview in Google Book Search).
- ^ SP Fricker: Metal Compounds in Cancer Therapy . Springer Science & Business Media, 2012, ISBN 978-94-011-1252-9 , pp. 138 ( limited preview in Google Book search).