Samarium (III) sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
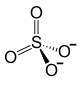
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Samarium (III) sulfate | |||||||||||||||
| other names |
Samarium sulfate |
|||||||||||||||
| Molecular formula | Sm 2 (SO 4 ) 3 · 8H 2 O | |||||||||||||||
| Brief description |
yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 733.03 g mol −1 (octahydrate) | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.93 g cm −3 (octahydrate) |
|||||||||||||||
| solubility |
slightly soluble in water, 26.7 g l −1 (20 ° C) (octahydrate) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Samarium (III) sulfate (Sm 2 (SO 4 ) 3 ) is a salt of the rare earth metal samarium with sulfuric acid . As an octahydrate, it forms yellow crystals.
Individual evidence
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-86.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)
