Structural viscosity
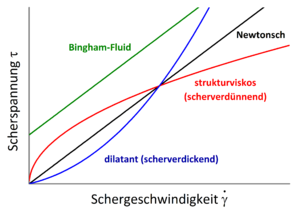
Structural viscosity , also known as shear thinning , is the property of a fluid to show a decreasing viscosity at high shear forces . That is, the stronger the shear that acts on the fluid, the less viscous (viscous) it becomes. In English, such a fluid is therefore called shear-thinning , in German it is called shear thinning , which is occasionally used as a synonym for structurally viscous.
The decrease in viscosity is caused by a structural change in the fluid, which ensures that the individual fluid particles (e.g. polymer chains ) slide past each other better.
Since the viscosity in a structurally viscous fluid does not remain constant with increasing shear, it is classified as a non- Newtonian fluid . Other fluids from this classification have u. a. following properties:
- Dilatancy : the opposite of structural viscosity, i.e. H. Viscosity increased with increasing shear, also called shear thickening
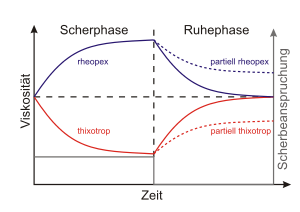
- Thixotropy : viscosity decrease with the duration of exposure
- Rheopexy : viscosity increase with the duration of exposure
Examples of pseudoplastic fluids
- In polymer solutions and melts , the individual polymer chains are intertwined (= hooked). With increasing shear force, these entanglements loosen and the viscosity decreases. This effect plays a major role in the processing of thermoplastics .
- Non-dripping wall paint does not drip from the roll because the shear is small and therefore the toughness or viscosity is high, whereas it is easy to apply to the wall because the thin layer between the wall and the roll causes a large shear and therefore the viscosity is low is.
- Blood has a high viscosity at low shear forces. With stronger shear forces, the red blood cells deform into rather elongated structures. This allows the blood to flow better through the small veins.
- Associative materials are systems in which small molecules come together through physical interactions, for example hydrogen bonds or ion-dipole interactions - to form supramolecular systems . Shear breaks these weak bonds (compared to covalent bonds ), which lowers the viscosity. The special feature here is that the bonds only recede completely after a certain material-specific time (→ thixotropy ). Technically important representatives are ionomers .
- The slime from land snails is structurally viscous, otherwise they would not be able to move.
Yield point
A special feature occurs in systems with fillers . For example, blood can be understood as a suspension of water and various solids (red blood cells). If the solids accumulate in a certain formation, the flow behavior changes. At low shear rates, a strong flow restriction occurs, which is referred to as the flow limit . A simple representative of these substances is the Bingham fluid .
The yield point is important, for example, when trying to take some ketchup from a ketchup bottle that has not been moved for a long time. Either the applied shear stress is very low, then nothing happens, or the yield point is overcome by heavy stress, and then far too much comes out.