Senecioaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
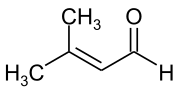
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Senecioaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 8 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 84.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.872 g ml −1 (25 ° C) |
|||||||||||||||
| boiling point |
133-135 ° C |
|||||||||||||||
| solubility |
110 g l −1 (20 ° C) |
|||||||||||||||
| Refractive index |
1.462 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Senecioaldehyde ( 3-methylcrotonaldehyde ) is an organic compound with the empirical formula C 5 H 8 O from the group of aldehydes with an additional C = C double bond , more precisely the alkenals . It is also one of the α, β-unsaturated carbonyl compounds.
It is made from isoprenol by air oxidation on a silver catalyst.
Individual evidence
- ↑ a b c d e f data sheet 3-Methylcrotonaldehyde from Sigma-Aldrich , accessed on July 28, 2019 ( PDF ).
- ↑ K. Hüsnü, Can Başer, Gerhard Buchbauer: Handbook of Essential Oils: Science, Technology, and Applications , 2nd edition, Boca Raton, 2016, ISBN 978-1-4665-9046-5 , p. 192 ( limited preview in Google Book Search).
- ↑ Patent application WO2008037693 : Continuous process for the production of citral. Registered on September 26, 2006 , published on April 3, 2008 , applicant: BASF SE, inventor: Günter Wegner, Gerd Kaibel, Jörg Therre, Werner Aquila and Hartwig Fuchs.


