Delayed boiling
Delayed boiling is the term on the one hand for the phenomenon that liquids can be heated above their boiling point under certain conditions without boiling , and on the other hand the term for the sudden boiling itself.
description
The delayed boiling effect occurs most frequently with water or aqueous solutions. Water can be heated to 110 ° C without boiling and thus without the formation of water vapor bubbles.
This state is metastable and therefore dangerous, since even a slight shock can form a large gas bubble within a very short time , which then escapes explosively from the vessel and often entrains boiling liquid. This occurs especially in narrow, tall vessels that offer little space for a foaming liquid. One example is test tubes . Smooth, even vessel walls, a low degree of mixing and a high degree of purity of the liquid promote delayed boiling.
root cause
The lack of a nucleation nucleus , i.e. a smooth, homogeneous surface of the vessel and a pure, gas and particle-free liquid, acts as a kinetic obstacle. The formation of a stable, gaseous phase is prevented and the liquid can overheat beyond its boiling point, i.e. delayed boiling .
The vapor pressure inside the first small vapor bubbles must be greater than the sum of the ambient pressure, the additional pressure caused by the surface tension and the pressure of the liquid column above the bubble. The surface tension leads to a particularly high pressure in small bubbles, so the onset of boiling is delayed.
After all, after a certain temperature a gas bubble forms and rises due to the static buoyancy . As the water column above it decreases, the hydrostatic pressure on it decreases. Liquid can evaporate further into the gas bubble and it increases in volume. This also reduces the pressure caused by the surface tension and the vapor pressure and the boiling temperature continue to decrease. The more overheated the liquid, the faster this process takes place. The gas bubble expands explosively due to the rapid supply of steam ( physical explosion ; see also fat explosion ) and presses the liquid loaded on it upwards. This leads to violent splash or spill over. The boiling point in this region suddenly drops to normal.
As to the cause of a delay in boiling (in the sense of overheating above the boiling point), there is also the theory that cohesive forces to air bubbles (dissolved air) absorb the energy and thereby make overheating possible.
hazards
Delayed boiling subsequently leads to the heated liquid being thrown out suddenly and can cause scalds to people standing nearby or severe burns if acids are overheated . Individual drops can get on the heat source and also evaporate suddenly and as a result their ingredients can burn.
Countermeasures
In the household, no countermeasures are normally required when cooking on the stove, as the heat-emitting surfaces of normal cookware are sufficiently rough due to burnt-on food residues or limescale deposits. Milk monitors can prevent delayed boiling when boiling milk (but not foaming over due to too much energy being supplied). There may be a delay in boiling, especially when heating liquids in a microwave oven .
In laboratory practice, the delay in boiling can be prevented by using suitable larger vessels, for example roughened evaporation dishes and frequently used (thus scratched inside) larger flasks instead of thin, new test tubes. Added to this is the very careful heating of the liquid and the avoidance of immobile resting positions during the heating process. Both of these factors mean that vessels are swiveled over the flame of a Bunsen burner , or , in the case of a hot plate, a magnetic stirrer is used or a rotary evaporator is used. In all cases, however, slow and even heating is of the utmost importance, which is why one should avoid particularly strongly preheated heating surfaces.
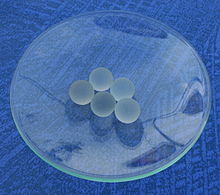
If there is an increased risk of delayed boiling, so-called boiling pearls or boiling stones (not to be confused with the mineralogical designation boiling stones ) are used. They consist of porous, largely inert material such as glass powder, glass beads with a rough surface, broken glass, shards of clays or silicate rocks. On the one hand, their rough surface interferes with the formation of a homogeneous molecular arrangement in the liquid; on the other hand, the air bound in the pores expands when heated and acts as a boiling nucleus when it rises. In order to trap air again, you should not use boiling stones again after the boiling has ended, but dry them in a drying cabinet. When vapor bubbles rise, small boiling stones are carried away in the liquid; the re-impact on the glass bottom leads to further nucleation nuclei. Boiling stones, however, are not very useful for vacuum distillation because they release the air they contain when evacuating. Usually, in addition to using small boiling stones, the glass surface is roughened with a glass rod.
You can also place a boiling rod in the solution to be heated. This is a glass rod with an open cavity on the underside.
Boiling capillaries are especially used for distillations in a vacuum . These are very thinly drawn glass tubes at the lower end, the lower end of which should touch the bottom of the flask and through which air or possibly a mostly inert gas ( noble gases ) is sucked in.
Another method of greatly reducing the boiling delay in distillations is to use a rotary evaporator . Vigorous stirring also helps with small amounts.
When heating test tubes , the heating is regularly interrupted and the contents are shaken through by briefly tapping the test tube in the middle section with your ring finger, middle finger and index finger without the liquid spilling over or burning on the hot glass.
Safety instructions for working in the laboratory
Warming with the risk of delayed boiling should generally always take place with the fume cupboard locked and only with the use of protective clothing (protective goggles, rubber gloves, as little skin as possible). The vessel should be used in such a way that no further damage can be caused despite delayed boiling (even overheated water is dangerous!). The opening of vessels should never be directed at one's own field of vision or at other people.
If you have forgotten to add boiling stones, you can only do so if you are absolutely sure that the boiling point has not yet been reached. Adding boiling stones to a liquid that is already overheated leads to explosive boiling.
The effect of boiling stones diminishes over time, which is why they should not be relied on and newly broken boiling stones have to be added to each heating process.
Demarcation
The boiling over of milk, pasta cooking water or jam can be due to delayed boiling, but in most cases it is due to the strong foaming of flocculated protein compounds . The gas bubbles formed are stabilized by special ingredients, so they do not burst and run over the edge. When boiling milk, this can be prevented by using a milk monitor.
Web links
- UiW-Lexikon: Sieden ( Memento of December 27, 2004 in the Internet Archive )
- Superheating and microwave ovens and others video of superheated water in a microwave oven with subsequent boiling delay
- Superheated Water in a Microwave Oven ( Memento from October 15, 2006 in the Internet Archive ) Series of video recordings of the reaction of a vessel with superheated water to vibrations by Louis A. Bloomfield, physics professor at the University of Virginia. From experiment 13 onwards, the energy that can be released in the event of delayed boiling is shown with impressive force. ( RealVideo , approx. 5.34 MB, English)
Individual evidence
- ↑ http://www.lassp.cornell.edu/sethna/Nucleation/ James P. Sethna: Critical Droplets and Nucleation , Cornell University 2002, accessed April 29, 2020
- ^ Heinrich Greinacher: Supplements to Experimental Physics. Springer-Verlag, 2013, ISBN 978-3-709-13492-4 , p. 59 ( limited preview in the Google book search).
- ^ Walter Wittenberger: Chemische Laboratoriumstechnik , Springer-Verlag, Vienna, New York, 7th edition, 1973, pp. 172-173, ISBN 3-211-81116-8 .
- ↑ Why does milk boil over quickly? Toni Fröhlich, Max Planck Institute for Dynamics and Self-Organization