Silica
Silica is the name for a refractory ceramic building material consisting of SiO 2 . Nowadays its use is largely limited to the vaulting of glass tubs and the lattice of blast heaters in blast furnaces. The material is of particular interest to materials engineers , glass and steel manufacturers.
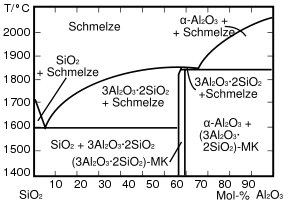
Position in the two- component system SiO 2 -Al 2 O 3
The melting temperature of pure SiO 2 initially decreases with increasing aluminum oxide content (up to about 8% by mass) by more than 100 K. For high heat resistance, an aluminum oxide content must therefore be avoided, even in small amounts. The proportion should be well below 1%.
Mineral phases
- Cristobalite 35-45%
- Tridi with 40-50%
- Quartz 0.5-3%
- Silica glass 4–8% (X-ray amorphous, possible impurities are dissolved in it)
- Wollastonite approx. 5% (due to the addition of lime, sintering aid!)
raw materials
Requirement: SiO 2 that is as pure as possible . In no case aluminum oxide, not even in small amounts, since a eutectic is already a few percent Al 2 O 3 .
- Rock quartzite : worse, as the conversion of quartz to cristobalite or tridymite is slow due to the low specific surface area. Relatively cheap to buy.
- Boulders / cement quartzite: better, since quartz is rapidly converted to cristobalite or tridymite due to the high specific surface area.
The raw materials should be as gray as possible and have no black or red inclusions (reference to iron and organic matter). No foliation should be evident as this is an indication of mica containing aluminum oxide.
Necessary laboratory tests:
- Loss on ignition due to organic components, later pores
- chemical analysis: Al 2 O 3 , FeO, Fe 2 O 3 , alkalis
- Conversion behavior
Shaping
Classic ceramic shaping: dry pressing , slip casting , etc.
However, the addition of clay has to be dispensed with, since it would add impurities (Al 2 O 3 , alkalis , alkaline earths , Fe 2 O 3 and others). In the case of silica stones, the chemical purity is elementary, since they are only sufficiently thermally resistant in an absolutely pure form ( eutectic reactions, formation of a melt phase).
To compensate for the lack of clay and thus the lack of dry strength, the stones are consolidated differently. It has been found that sulphite waste liquor (waste product from the paper industry: Ca (SO 3 ) 2 with organic components) is suitable as a binder for silica bricks.
Sintering of silica
Sintering silica is very problematic in many ways.
- The sulphite waste liquor is thermally decomposed. It occurs sulfur dioxide (SO 2 ) is made, which must be filtered from the flue gas. There is gypsum that is used in the construction industry.
- The conversion of quartz into tridymite results in a volume expansion of 14.5%, from that of quartz into cristobalite one of 17%. Therefore, the shaped stones tend to crack easily. Consequence: It has to be heated up very slowly. And it has to be cooled down just as slowly. At temperatures above 700 ° C, the heating or cooling rate is irrelevant, since all changes in the modification of the SiO 2 system have taken place there.
- The lime in the batch serves as a sintering aid. At 600 ° C, lime CaCO 3 degases to CaO and combines directly with the SiO 2 from the residual material to form wollastonite CaSiO 3 and thus creates the binding phase between the silica grains.
- Firing between 1350 ° C and 1500 ° C depending on the ratio of rock quartzite / boulder quartzite
When sintering, you generally have to weigh up between the following variants:
- slow heating: avoid cracks due to modification , high costs
- fast sintering: save energy, risk of cracking
- long hold time / short hold time: Degree of conversion of quartz to cristobalite / tridymite
Properties of silica stones
- good resistance to acid slags
- poor resistance to basic slags
- Application temperature: 1550-1600 ° C
- very sensitive to reduction: (only above 1500 ° C)
- very small softening interval (depending on the impurities, the interval can be skewed downwards)
- Very high coefficient of thermal expansion (CTE) below 700 ° C due to many changes in modification with strong volume expansion
- very low CTE above 700 ° C
- below 700 ° C very poor thermal shock resistance (TWB), change due to modification
Typical areas of application
- Vaults of glass tubs (although basic attack (alkalis), infiltration, saturation, solidification in the back stone layers, further infiltration stopped)
- Coke oven construction
- Wind heater (blast furnace, heating of the blown air)
- Filler for tires to reduce rolling resistance
swell
- Gerald Routschka (Ed.): Article Fred Brunk, paperback fire-resistant materials, Vulkan-Verlag, ISBN 3-8027-3150-6 .