Tridymite
| Tridymite | |
|---|---|
|
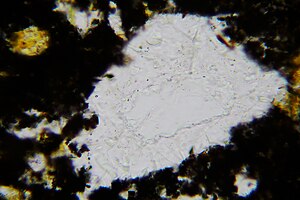
Tridymite in the form of ultra-thin, colorless and pseudo-hexagonal panels (image size: 1.1 mm) Find location: tub heads, Ochtendung, Eifel, Germany |
|
| General and classification | |
| other names |
Asmanite |
| chemical formula | SiO 2 |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.DA.10 ( 8th edition : IV / D.01) 75.01.02.01 |
| Crystallographic Data | |
| Crystal system | orthorhombic |
| Crystal class ; symbol | orthorhombic-disphenoidic; 222 |
| Space group | see crystal structure |
| Lattice parameters | see crystal structure |
| Formula units | see crystal structure |
| Physical Properties | |
| Mohs hardness | 7th |
| Density (g / cm 3 ) | measured: 2.25 to 2.28; calculated: [2.28] |
| Cleavage | {0001} indistinct, {1010} imperfect |
| Break ; Tenacity | clamshell; brittle |
| colour | colorless, white, yellowish white, gray |
| Line color | White |
| transparency | transparent to translucent |
| shine | Glass gloss |
| Crystal optics | |
| Refractive indices |
n α = 1.468 to 1.482 n β = 1.470 to 1.484 n γ = 1.474 to 1.486 |
| Birefringence | δ = 0.006 |
| Optical character | biaxial positive |
| Axis angle | 2V = 40 to 86 ° (measured); 50 to 72 ° (calculated) |
Tridymite (also asmanite ) is an orthorhombic high-temperature modification of quartz , a crystalline form of silicon dioxide . Tridymite has only been generally recognized as a stable phase of SiO 2 since the 1960s . Another high temperature modification of quartz is cristobalite .
Etymology and history
In July 1867, Gerhard vom Rath received two samples of trachytic porphyry from the Cerro San Cristóbal mountain near Pachuca de Soto (Hidalgo, Mexico). In these samples, in addition to the already known minerals iron luster ( hematite ) and hornblende, he also discovered a previously unknown, colorless mineral with an unusual crystal shape. This turned out to be a characteristic growth of triplets , due to which Gerhard vom Rath called the mineral tridymite after the Greek word τρίδυμο [tridymo] for triple.
classification
In the now outdated, but still in use 8th edition of the mineral classification according to Strunz , the tridymite belonged to the mineral class of "oxides and hydroxides" and there to the department of "oxides with metal: oxygen = 1: 2", where it, together with coesite , cristobalite , Melanophlogite , mogánite , opal , quartz and stishovite the "quartz group" with the system no. IV / D.01 .
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also assigns the tridymite to the class of "oxides and hydroxides" and there in the department of "metal: oxygen = 1: 2 and comparable "a. However, this section is further subdivided according to the size of the cations involved and the crystal structure or membership of a related mineral family, so that the mineral can be found according to its composition in the sub-section "With small cations: silica family" where it is only still forms the unnamed group 4.DA.10 together with Opal .
In contrast to Strunz's mineral systematics, the systematics of minerals used mainly in the English-speaking world, according to Dana , assigns the tridymite to the class of "silicates and germanates" and there in the department of " framework silicate minerals ". Here he is to be found as the only member of the unnamed group 75.01.02 within the subdivision “ Framework silicates: tetrahedral Si lattice, SiO 2 with [4] -coordinated Si ”.
Crystal structure
Tridymite has a monoclinic symmetry at room temperature, but there are a large number of structural modifications depending on the temperature, the complexity of which increases with decreasing temperature. The determination of these phases was completed in the 1980s. As the temperature drops, these phases are:
| Surname | Stability area | Space group | Lattice parameters |
|---|---|---|---|
| β-tridymite ( HP-tridymite ) | 465-1470 ° C | hexagonal, room group P 6 3 / mmc ( room group no.194) | a = 5.05 Å and c = 8.27 Å as well as 4 formula units per unit cell |
| Tridymite ( OC-tridymite ) | 180-350 ° C | orthorhombic, C 222 1 (No. 20) | a = 8.74 Å; b = 5.04 Å and c = 8.24 Å as well as 8 formula units per unit cell |
| OS tridymite | 150-190 ° C | Superstructure | |
| OP tridymite | 110-150 ° C | orthorhombic, P 2 1 2 1 2 1 (No. 19) | |
| MC tridymite | <110 ° C | monoclinic, Cc (No. 9) |
The most important phase is HP tridymite, which is the ideal high temperature phase of tridymite. It consists of the same layers of SiO 4 tetrahedra arranged in hexagonal rings. These layers are stacked on top of one another in an ABAB sequence, thus leaving uninterrupted tunnels.
When the temperature falls in the OC-Tridymite, a tilting of the tetrahedra initially leads to a shear of layers lying one behind the other. With the remaining modifications, the hexagonal rings are also deformed to ditrigonal and oval configurations, which alternate in a superstructure characteristic of the modification.
properties
The melting point of tridymite is 1670 ° C.
Under the microscope, thin section tridymite is inconspicuous due to its low light and birefringence, and it is difficult to distinguish from quartz in brightfield. The thin tabular or leafy, often twinned crystals appear under crossed polarizers in gray interference colors of the first order.
Modifications and varieties
Tridymite remains stable up to a maximum of 3 kbar and changes under normal pressure at 870 ° C to high quartz and at 1470 ° C to cristobalite.
Education and Locations
As a rather rare mineral formation, tridymite can sometimes be abundant at various sites, but overall it is not very common. In addition to its occurrence in acidic, SiO 2 -rich volcanic rocks, it is also found in contact metamorphic rocks of the sanidinite facies . Around 300 sites are known to date (as of 2012). In addition to its type locality Cerro San Cristóbal in Hidalgo , the mineral was also found in Mexico in the “La Esperanza Mine”, the “Barranca Mine” and the “Remedios Mine” in Durango , the “Santín Mine” in Guanajuato and the “Tocho Mine” San Luis Potosí is revealed.
In Germany, the mineral occurred mainly in Rhineland-Palatinate, where it could be found at many sites in the Eifel , including at Andernach , Daun , Ettringen and Mendig . Tridymite is also known from Baden-Württemberg, Bavaria, Hesse, North Rhine-Westphalia, Saxony and Thuringia.
In Austria, tridymite was found in the basalt quarry on Pauliberg in Burgenland as well as in the "Schlarbaum" quarry near Klausen ( Bad Gleichenberg ), on the Stradner Kogel and in the basalt quarry near Klöch in Styria.
Other locations include the Antarctic, Argentina, Azerbaijan, Australia, Bolivia, Brazil, Burundi, China, Ecuador, France, Greece, Iceland, India, Indonesia, Israel, Italy, Japan, Canada, Madagascar, Namibia, the Netherlands, in New Zealand, Oman, Papua New Guinea, Peru, Romania, Russia, Slovakia, Spain, South Africa, Tajikistan, the Czech Republic, Turkey, Hungary, the United Kingdom (Great Britain) and the United States of America (USA).
Tridymite was also found in the basalt of the lunar sea " Mare Tranquillitatis ", from which the crew of the first Apollo 11 lunar landing mission brought some rock samples, as well as in the basalt of the Fra Mauro highlands, which was visited by the crew of the Apollo 14 mission.
In June 2016, NASA announced that the Mars rover Curiosity had also found tridymite on Mars.
See also
literature
- Tadayuki Hirose, Kuniaki Kihara, Masayuki Okuno, Syuhei Fujinami, Keiji Shinoda: X-ray, DTA and Raman studies of monoclinic tridymite and its higher temperature orthorhombic modification with varying temperature . In: Journal of Mineralogical and Petrological Sciences . tape 100 , 2005, pp. 55–69 ( rruff.info [PDF; 1.4 MB ; accessed on July 7, 2018]).
- Peter J. Heaney: Structure and chemistry of the low-pressure silica polymorphs . In: Reviews in Mineralogy and Geochemistry . tape 29 , no. 1 , 1994, p. 1-40 .
- M. Wennemer, AB Thompson: Tridymite polymorphs and polytypes . In: Swiss mineralogical and petrographic messages . tape 64 , 1984, pp. 335-353 .
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 450 .
Web links
- Mineral Atlas: Tridymite
- Mindat - Tridymite (English)
Individual evidence
- ↑ Tridymite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 69 kB ; accessed on July 7, 2018]).
- ↑ a b c d e f Mindat - Tridymite
- ↑ Karl Friedrich Rammelsberg: The chemical nature of meteorites . In: Treatises of the Royal Academy of Sciences in Berlin . 1879, p. 8–9 ( http://www.archive.org/stream/abhandlungenderk1879deut#page/n43/mode/2up/search/Asmanit available online at archive.org [accessed July 7, 2018]).
- ↑ Gerhard vom Rath : About the tridymite, a new crystallized modification of silicic acid . In: Annals of Physics and Chemistry . tape 135 , 1868, pp. 437–454 ( rruff.info [PDF; accessed July 7, 2018]).
- ^ A b Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 205 .
- ^ William Alexander Deer, Robert Andrew Howie, Jack Zussman: An Introduction to the Rock Forming Minerals . Longman Scientific & Technical, London 1966, ISBN 978-0-582-44210-8 , pp. 340-355 .
- ↑ a b Hans Pichler, Cornelia Schmitt-Riegraf: Rock -forming minerals in thin sections . 2nd Edition. Enke, Stuttgart 1993, ISBN 3-8274-1260-9 , pp. 66 .
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 426-429 .
- ↑ Mindat - Localities for Tridymite
- ↑ mars.nasa.gov: NASA Scientists Discover Unexpected Mineral on Mars - Mars Science Laboratory. In: mars.jpl.nasa.gov. Retrieved June 24, 2016 .