Amphibole group
| Amphibole | |
|---|---|
|
Amphibole (edenite) from Bancroft, Hastings County, Ontario, Canada Size: 2.2 × 1.9 × 1.1 cm |
|
| General and classification | |
| other names |
Hornblende |
| chemical formula | see single minerals |
|
Mineral class (and possibly department) |
Chain silicates and band silicates ( inosilicates ) |
|
System no. to Strunz and to Dana |
9.DD. until 9.DE. ( 8th edition : VIII / F.7 to VIII / F.12) 65.00.00 |
| Crystallographic Data | |
| Crystal system | monoclinic or orthorhombic |
| Crystal class ; symbol | 2 / m or 2 / m 2 / m 2 / m |
| Physical Properties | |
| Mohs hardness | 5 to 6 |
| Density (g / cm 3 ) | 3 to 3.6 |
| Cleavage | completely according to (110) in monoclinic amphiboles. Perfect according to (210) and bad according to (100) in orthorhombic amphiboles. 2 good cleavages parallel c form an angle of 55 ° or 125 ° |
| Break ; Tenacity | mostly brittle |
| colour | variable, see individual minerals |
| Line color | variable, see individual minerals |
| transparency | transparent to opaque |
| shine | Glass gloss |
The amphibole group (short: amphiboles) comprises silicates which are structurally characterized by double chains of corner-linked SiO 4 tetrahedra and whose composition complies with the following generalized empirical formula:
A 0-1 B 2 C 5 T 8 O 22 (OH) 2 .
In this structural formula, the capital letters A, B, C and T represent different positions in the amphibole structure. They are occupied by the following cations:
- A : vacancies, Na + , K + , Ba 2+ , Sr 2+ , Ca 2+ , Li +
- B : Ca 2+ , Na + , Mg 2+ , Fe 2+ , Mn 2+ , Li + , Sr 2+ , Ba 2+ , Mn 2+ , Fe 2+ , Co 2+ , Ni 2+ , Zn 2 + , Mg 2+ , Pb 2+ , Cu, Zr, Mn 3+ , Cr 3+ , V, Fe 3+
- C : Mg 2+ , Fe 2+ , Mn 2+ , Li + , Al 3+ , Zn 2+ , Ni 2+ , Co 2+ , Ti 4+ , Fe 3+ , V, Cr 3+ , Mn 3+ , Zr
- T : Si 4+ , Al 3+ , Ti 4+
Instead of the hydroxyl group (OH), amphiboles also contain F - , Cl - , O 2−
The dominant cations on the individual positions are highlighted in bold.
Amphiboles are the mineral group with the greatest chemical variability. This is one of the reasons why amphiboles appear in many different parageneses and geological environments around the world. They are an important component of both igneous and metamorphic rocks of various compositions and formation conditions.
Various minerals in this group are called asbestos .
Classification and nomenclature
Due to the chemical and crystallographic complexity of the amphiboles, a uniform nomenclature was no easy undertaking. In 1997, the "Subcommittee on Amphiboles" of the "International Mineralogical Association, Commission on New Minerals and Mineral Names" was continuously simplified until 2003 and supplemented by the discovery of new Li, Na amphiboles with a fifth group. The standardization is based on the content of the various elements in the individual positions (A, B, C, T), whereby the occupation of the B position is decisive for the subdivision of the amphiboles.
- Group: Magnesium-Iron-Manganese-Lithium-Amphiboles: Mg + Fe + Mn + Li ≥ 1.5
- Group: Calcium amphiboles: Mg + Fe + Mn + Li <1.5 and Ca + Na ≥ 1.00 and Na <0.50
- Group: Sodium-Calcium-Amphiboles: Mg + Fe + Mn + Li ≤ 0.50 and Ca + Na ≥ 1.00 and 0.50 ≤ Na ≤ 1.50
- Group: Alkali amphiboles: Mg + Fe + Mn + Li ≤ 0.50 and Na ≥ 1.50
- Group: Na-Ca-Mg-Fe-Mn-Li-amphiboles: 0.50 ≤ Mg + Fe + Mn + Li ≤ 1.50 and 0.50 ≤ Na + Ca ≤ 1.50
In these five groups, 78 basic names for amphiboles are defined. Deviations from the compositions listed below, e.g. B. by incorporating further cations not specified here in the crystal lattice such as chromium, lead, potassium ..., is taken into account by name prefixes and preceding adjectives.
Prefixes in amphibole names
| Amphibole name prefixes | ||
|---|---|---|
| prefix | meaning | use |
| Titano | Ti> 0.50 | all groups, without Kaersutit |
| Alumino | VI Al> 1.00 | calcium and sodium-calcium amphiboles only |
| Ferri | Fe 3+ > 1.00 | All groups except sodium amphiboles |
| Chromio | Cr> 1.00 | All groups |
| Mangani | Mn 3+ > 1.00 | All groups except Kornit and Ungarettiit |
| Mangano | 1.0 <Mn 2+ <2.99 | All groups except Kornit and Ungarettiit |
| Permangano | 3.00 <Mn 2+ <4.99 | All groups except Kornit and Ungarettiit |
| Ferro | Fe 2+ > Mg | All groups |
| Magnesio | Fe 2+ <Mg | All groups |
| Zinko | Zn 2+ > 1.00 | All groups |
| potassium | K> 0.50 | All groups |
| sodium | Na> 0.50 | group 1 only |
| Chloro | Cl> 1.00 | All groups |
| Fluoro | F> 0.50 | All groups |
Prefixes indicate essential substitutions and are an integral part of the name. They are placed directly in front of the base name, in the same word or separated by a hyphen.
Preceding adjectives describe subordinate variations in the composition. They are placed in front of the base name as an adjective without a hyphen (containing barium, containing boron, containing lead, containing nickel, etc.).
In the structural formulas given below, the atoms in brackets can be substituted in any mixture , but they are always in the same relationship to the other atom groups.
Magnesium-iron-manganese-lithium amphiboles
If more than 1.5 of the 2 lattice sites in the B position are occupied by Mg 2 ++ , Fe 2 ++ , Mn 2 ++ or Li + , it is an amphibole of the Mg-Fe-Mn-Li group . Amphiboles of this group occur with both orthorhombic and monoclinic symmetry .
Orthorhombic amphiboles:
Most orthorhombic amphiboles crystallize in the space group Pnma. The occurrence of the space group Pnmn can be identified in the name by the prefix Proto.
Anthophyllite series:
This series includes amphiboles with the composition Na x Li z (Fe 2+ , Mg, Mn) 7-yz Al y (Si 8-x-y + z Al x + yz ) O 22 (OH, F, Cl) 2 with Si > 7.0 and Li <1.0. The following end links form the boundaries of the anthophyllite compositions:
- Anthophyllite Mg 7 Si 8 O 22 (OH) 2
- Ferro-anthophyllite Fe 7 Si 8 O 22 (OH) 2
- Sodium anthophyllite NaMg 7 Si 7 AlO 22 (OH) 2
- Sodium Ferro-Anthophyllite NaFe 7 Si 7 AlO 22 (OH) 2
Gedrit series:
Compositions of this series are described by the same structural formula as the anthophyllite series, but with Si <7.0. The series contains the following end links:
- Ferrogenite Fe 5 Al 2 (Si 6 Al 2 ) O 22 (OH) 2
- Gedrit Mg 5 Al 2 (Si 6 Al 2 ) O 22 (OH) 2
- Sodium ferrogenite NaFe 6 Al (Si 6 Al 2 O 22 ) (OH) 2
- Sodium drite NaMg 6 Al (Si 6 Al 2 ) O 22 (OH) 2
Holmquistit series:
This series contains amphiboles with the composition [Li 2 (Fe 2+ , Mg) 3 (Fe 3+ , Al) 2 ] Si 8 O 22 (OH, F, Cl) 2 with Li> 1.0.
- Holmquistite (Li 2 Mg 3 Al 2 ) Si 8 O 22 (OH) 2
- Ferroholmquistite (Li 2 Fe 3 Al 2 ) Si 8 O 22 (OH) 2
Monoclinic amphiboles
Cummingtonite-Grunerite series:
Amphiboles of this series satisfy the general formula (Mg, Fe, Mn, Li) 7 Si 8 O 22 (OH) 2 with Li <1.0.
- Cummingtonite Mg 7 Si 8 O 22 (OH) 2
- Grunerite Fe 7 Si 8 O 22 (OH) 2
- Manganocummingtonite Mn 2 Mg 5 Si 8 O 22 (OH) 2
- Manganogrunerite Mn 2 Fe 5 Si 8 O 22 (OH) 2
- Permanganogrunerite Mn 4 Fe 3 Si 8 O 22 (OH) 2
Klinoholmquistit series:
This series is described by the structural formula [Li 2 (Fe 2+ , Mg, Mn) 3 (Fe 3+ , Al) 2 ] Si 8 O 22 (OH, F, Cl) 2 with more than 1.0 Li on the B position and, crucially, less than 0.5 Li in the C position.
- Clinoferroholmquistite (Li 2 Fe 3 Al 2 ) Si 8 O 22 (OH) 2
- Clinoholmquistite (Li 2 Mg 3 Al 2 ) Si 8 O 22 (OH) 2
- Ferri-Klinoferroholmquistite (Li 2 Fe 2+ 3 Fe 3+ 2 ) Si 8 O 22 (OH) 2
- Ferri-Klinoholmquistit (Li 2 Mg 2+ 3 Fe 3+ 2 ) Si 8 O 22 (OH) 2
Pedrizit-Ferropedrizit series:
This series is described by the structural formula NaLi 2 [Li (Fe 2+ , Mg, Mn) 2 (Fe 3+ , Al) 2 ] Si 8 O 22 (OH, F, Cl) 2 with more than 1.0 Li on the B position and, crucially, more than 0.5 Li in the C position.
- Sodium Pedricity : NaLi 2 (LiMg 2+ 2 Fe 3+ Al) Si 8 O 22 (OH) 2
- Sodium ferropedicity: NaLi 2 (LiFe 2+ 2 Fe 3+ Al) Si 8 O 22 (OH) 2
Calcium amphiboles
This group, often referred to synonymously as hornblende, includes monoclinic amphiboles with more than 1.5 Ca in the B position. A further subdivision can be made based on the occupation of the A position. The following end members belong to this group:
Calcium amphiboles with less than 0.5 (Na, K) in the A position:
- Tremolite Ca 2 Mg 5 Si 8 O 22 (OH) 2
- Ferro-actinolite Ca 2 Fe 2+ 5 Si 8 O 22 (OH) 2
- Magnesio hornblende Ca 2 [Mg 4 (Al, Fe 3+ )] (Si 7 Al) O 22 (OH, F) 2
- Ferro-hornblende Ca 2 [Fe 2+ 4 (Al, Fe 3+ )] (Si 7 Al) O 22 (OH, F) 2
- Tschermakit Ca 2 (Mg 3 Fe 3+ Al) (Si 6 Al 2 ) O 22 (OH) 2
- Ferrotschermakit Ca 2 (Fe 2+ 3 Fe 3+ Al) (Si 6 Al 2 ) O 22 (OH) 2
- Aluminochermakite Ca 2 (Mg 3 Al 2 ) (Si 6 Al 2 ) O 22 (OH) 2
- Alumino-Ferrotschermakit Ca 2 (Fe 2+ 3 Al 2 ) (Si 6 Al 2 ) O 22 (OH) 2
- Ferrite shearite Ca 2 (Mg 3 Fe 3+ 2 ) (Si 6 Al 2 ) O 22 (OH) 2
- Ferri-Ferrotschermakit Ca 2 (Fe 2+ 3 Fe 3+ 2 ) (Si 6 Al 2 ) O 22 (OH) 2
Calcium amphiboles with more than 0.50 Ca in the A position:
- Cannilloite CaCa 2 (Mg 4 Al) (Si 5 Al 3 ) O 22 (OH) 2
Calcium amphiboles with more than 0.5 (Na, K) in the A position:
- Edenite NaCa 2 Mg 5 (Si 7 Al) O 22 (OH) 2
- Ferro-Edenit NaCa 2 Fe 2+ 5 (Si 7 Al) O 22 (OH) 2
- Pargasite NaCa 2 Mg 4 Al (Si 6 Al 2 ) O 22 (OH) 2
- Ferro-Pargasite NaCa 2 Fe 2+ 4 Al (Si 6 Al 2 ) O 22 (OH) 2
- Magnesiohastingsite NaCa 2 Mg 4 Fe 3+ (Si 6 Al 2 ) O 22 (OH) 2
- Hastingsite NaCa 2 Fe 2+ 4 Fe 3+ (Si 6 Al 2 ) O 22 (OH) 2
- Magnesiosadanagaite NaCa 2 [Mg 3 (Fe 3+ , Al) 2 ] (Si 5 Al 3 ) O 22 (OH) 2
- Sadanagaite NaCa 2 [Fe 2+ 3 (Fe 3+ , Al) 2 ] (Si 5 Al 3 ) O 22 (OH) 2
Calcium amphiboles with more than 0.5 (Na, K) in the A position and more than 0.50 Ti:
- Kaersutite NaCa 2 (Mg 4 Ti) (Si 6 Al 2 ) O 22 (OH) 2
- Ferrokaersutite NaCa 2 (Fe 2+ 4 Ti) (Si 6 Al 2 ) O 22 (OH) 2
Sodium-calcium amphiboles
This group includes monoclinic amphiboles with more than 1 (Ca, Na) in the B position ( B (Ca, Na)> = 1.0) and Na levels in the B position between 0.50 and 1.50 apfu (atoms per formula unit). A further subdivision is based on the occupation of the A position. The following end links belong to this group:
Sodium-calcium amphiboles with more than 0.50 (Na, K) in the A position (Richterit-Katophorit-Taramit):
- Richterite Na (CaNa) Mg 5 Si 8 O 22 (OH) 2
- Ferrorichterite Na (CaNa) Fe 2+ 5 Si 8 O 22 (OH) 2
- Magnesiocatophorite Na (CaNa) (Mg 4 (Al, Fe 3+ )) (Si 7 Al) O 22 (OH) 2
- Katophorite Na (CaNa) (Fe 2+ 4 (Al, Fe 3+ )) (Si 7 Al) O 22 (OH) 2
- Magnesiotaramite Na (CaNa) (Mg 3 AlFe 3+ ) Si 6 Al 2 O 22 (OH) 2
- Tara with Na (CaNa) (Fe 2+ 3 AlFe 3+ ) Si 6 Al 2 O 22 (OH) 2
- Potassium aluminotaramite K (CaNa) (Fe 2+ 3 Al 2 ) Si 6 Al 2 O 22 (OH) 2
Sodium-calcium amphiboles with less than 0.50 (Na, K) in the A-position (Winchit-Barroisit):
- Winchit (CaNa) Mg 4 (Al, Fe 3+ ) Si 8 O 22 (OH) 2
- Ferrowinchite (CaNa) Fe 2+ 4 (Al, Fe 3+ ) Si 8 O 22 (OH) 2
- Barroisite (CaNa) (Mg 3 AlFe 3+ ) (Si 7 Al) O 22 (OH) 2
- Ferrobarroisite (CaNa) (Fe 2+ 3 AlFe 3+ ) (Si 7 Al) O 22 (OH) 2
- Aluminobarroisite (CaNa) (Mg 3 Al 2 ) (Si 7 Al) O 22 (OH) 2
- Alumino-Ferrobarroisite (CaNa) (Fe 2+ 3 Al 2 ) (Si 7 Al) O 22 (OH) 2
- Ferribarroisite (NaCa) (Mg 3 Fe 3+ 2 ) Si 7 AlO 22 (OH) 2
- Ferri-Ferrobarroisite (CaNa) (Fe 2+ 3 Fe 3+ 2 ) (Si 7 Al) O 22 (OH) 2
Alkali amphiboles
This group includes monoclinic amphiboles with more than 1.50 Na in the B position. This group is subdivided on the basis of the Li and Mn contents as well as the Na contents in the A position.
Alkali amphiboles with less than 0.5 Li, (Mn 2+ + Mn 3+ ) <( VI Al + Fe 3+ + Fe 2+ + Mg) and less than 0.50 Na + K on the A position: Glaucophane, Riebekit
- Glaucophane Na 2 (Mg 3 Al 2 ) Si 8 O 22 (OH) 2
- Ferroglaucophane Na 2 (Fe 2+ 3 Al 2 ) Si 8 O 22 (OH) 2
- Magnesia beckite Na 2 (Mg 3 Fe 3+ 2 ) Si 8 O 22 (OH) 2
- Riebeckite Na 2 (Fe 2+ 3 Fe 3+ 2 ) Si 8 O 22 (OH) 2
Alkali amphiboles with less than 0.5 Li, (Mn 2+ + Mn 3+ ) <( VI Al + Fe 3+ + Fe 2+ + Mg) and more than 0.50 Na + K on the A position: Eckermannite, Arfvedsonite, Obertiit, Nyboeite
- Eckermannite NaNa 2 (Mg 4 Al) Si 8 O 22 (OH) 2
- Ferro-Eckermannite Na 3 (Fe 2+ 4 Al) Si 8 O 22 (OH) 2
- Magnesio-Arfvedsonite NaNa 2 (Mg 4 Fe 3+ ) Si 8 O 22 (OH) 2
- Arfvedsonite NaNa 2 (Fe 2+ 4 Fe 3+ ) Si 8 O 22 (OH) 2
- Obertiit NaNa 2 (Mg 3 Fe 3+ Ti) 2 Si 8 O 22 O 2
- Nybøit NaNa 2 Mg 3 Al 2 (Si 7 Al) O 22 (OH) 2
- Ferronybøit NaNa 2 (Fe 2+ 3 Al 2 ) (Si 7 Al) O 22 (OH) 2
- Ferrinyboit NaNa 2 (Mg 3 Fe 3+ 2 ) (Si 7 Al) O 22 (OH) 2
- Ferri-Ferronybøit NaNa 2 (Fe 2+ 3 Fe 3+ 2 ) (Si 7 Al) O 22 (OH) 2
Alkali amphiboles with less than 0.5 Li (Mn 2+ + Mn 3+ )> ( VI Al + Fe 3+ + Fe 2+ + Mg) and more than 0.50 Na + K on the A-position: Ungarettiite, Kozulite
- Ungarettiite NaNa 2 (Mn 2+ 2 Mn 3+ 3 Si 8 O 22 (OH) 2 )
- Kozulite Na 3 (Mn 2+ 4 (Fe 3+ , Al)) Si 8 O 22 (OH) 2
Alkali amphiboles with more than 0.5 Li: Leakeit, Kornit
- Leakeit NaNa 2 (Mg 2 Fe 3+ 2 Li) Si 8 O 22 (OH) 2
- Ferroleate NaNa 2 (Fe 2+ 2 Fe 3+ 2 Li) Si 8 O 22 (OH) 2
- Kornite (Na, K) Na 2 (Mg 2 Mn 3+ 2 Li) Si 8 O 22 (OH) 2
Sodium-Calcium-Magnesium-Iron-Manganese-Lithium-Amphibole
This group was only introduced by the IMA in 2003 in order to do justice to newer finds of amphiboles with unusually high contents of small divalent cations (Mg, Mn, Fe ...). Amphiboles of this group are characterized by occupation of the two B positions with 0.50 <(Mg, Fe 2+ , Mn 2+ , Li) <1.50 and 0.50 ≤ (Na, Ca) ≤ 1.50.
Group 5 amphiboles with more than 0.50 Li in the B position
- Ottoliniite (NaLi) (Mg 3 Fe 3+ Al) Si 8 O 22 (OH) 2
- Ferri- ottolinite (NaLi) (Fe 2+ 3 Fe 3+ Al) Si 8 O 22 (OH) 2
- Whittackerite Na (NaLi) (LiMg 2 Fe 3+ Al) Si 8 O 22 (OH) 2
- Ferriwhittackerite Na (NaLi) (LiFe 2+ 2 Fe 3+ Al) Si 8 O 22 (OH) 2
Group 5 amphiboles with less than 0.50 Li in the B position
The names of groups 1 to 4 are used here, supplemented with the prefix “Parvo” (Latin for 'small') to indicate the increased content of small cations in the B position.
structure
The great variation in the chemical composition of the amphiboles is explained by their structure. It has cation positions of very different sizes and shapes and thus offers space for a multitude of cations of different sizes and charges. In all these cation positions, the cations are surrounded by oxygen anions, if one disregards the smaller fluorine contents. The different positions differ in the number of surrounding anions (coordination number), their distance to the cation and their arrangement around the cation. In general, the following applies: The more anions surround a cation, the greater the mean distance from the cation position to the anions, the weaker the individual bonds and the greater the ionic character of the bonds.
The amphibole structure has cation positions with 4 different coordination numbers.
- Tetrahedral positions: 4 anions surround a cation in a tetrahedral shape. This position offers space for small cations, usually with a high charge (Si 4+ , Ti 4+ , Al 3+ ). The short cation-anion bonds have a high covalent content (atomic bonds). Atomic bonds are strongly directed. Therefore, the geometry of the binding atomic orbitals must match the arrangement of the surrounding anions as closely as possible.
- Octahedral positions: 6 anions surround a cation in an octahedral shape. This position offers space for medium-sized, mostly divalent and trivalent cations (Mg 2+ , Fe 2+ , Mn 2+ , Al 3+ , Fe 3+ ). The bonds are predominantly non-directional ionic.
- 8-fold coordinated places: 8 anions surround a cation in the form of a cubic antiprism. This position offers space for large monovalent to divalent cations (Na + , Ca 2+ ). The bonds are weak and ionic.
- 12-fold coordinated places: This position offers space for very large mono- to divalent cations (Na + , K + ). The bonds are very weakly ionic.
For the sake of clarity, the structural images only show the faces of these coordination polyhedra. The oxygen and cations themselves are not shown. The oxygen anions are on the corners of the polyhedra, the cations in the center of the polyhedra.
Silicate anion complex
The structural characteristic of all amphiboles is the double chain of SiO 4 tetrahedra with the empirical formula [Si 4 O 11 ] 6− . According to F. Liebau's silicate classification, amphiboles belong to the group of unbranched two double-chain silicates.
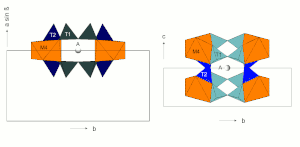
Silicon is surrounded by four oxygen atoms that form the corners of a tetrahedron with the Si 4+ cation in the middle . These SiO 4 tetrahedra are connected to ideally infinite chains via two oxygen atoms. Two such chains are connected to double chains via every second SiO 4 tetrahedron (see figure).
There are two structurally different SiO 4 tetrahedra. The tetrahedron T1 connects the two two-single chains to form a two-double chain and is connected to neighboring SiO 4 tetrahedra via three oxygen atoms . The tetrahedron T2 is only connected to neighboring SiO 4 tetrahedra via two oxygen atoms .
The SiO 4 tetrahedra are arranged in the double chains in such a way that they all point with a tetrahedron point in the same direction approximately perpendicular to the plane of the double chain. Accordingly, all tetrahedra with a face point in the opposite direction. The illustration opposite shows a section of a SiO 4 double double chain with a view of the tetrahedron base surfaces.
Coordination of the divalent and trivalent cations
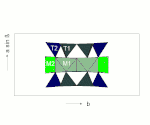
The C position from the structural formula given above comprises the three structurally distinguishable positions M1, M2 and M3. In these three positions the smaller cations (mainly Mg 2+ , Fe 2+ , Mn 2+ , Al 3+ ) are coordinated octahedrally by six oxygens.
The octahedra of the C-position are linked by common edges to ideally infinite octahedron bands. The B position is at the edges of the octahedron bands and creates the connection to neighboring SiO 4 tetrahedron double chains.
The B position (usually referred to as the M4 position in structural descriptions) is surrounded by eight oxygen atoms that lie on the corners of a distorted cubic or tetragonal antiprism. This position offers space for larger divalent cations such as Ca or Pb (all calcium amphiboles, e.g. tremolite), but can also be completely occupied by Mg and Fe (e.g. anthophyllite, grunerite).
The A-position is surrounded by 12 oxygen. It lies between two silicate double chains approximately above or below the center of the SiO 4 six rings (see figure) and thus creates a weak link between the I-beams (see following section). The double chains are not exactly one above the other, so that the oxygen configuration for the A position is very irregular.
I-beams
Two tetrahedral double chains are connected to the top and bottom of an octahedron band via their free tips. This sandwich-like structural unit is also referred to as an I-beam because of its cross-section reminiscent of the capital letter I.
These I-beams are connected to each other via the M4 and M2 octahedra, the edges of the silicate double chains being bound to the M4 and M2 octahedra of the neighboring I-beams.
Education and Locations
The iron-rich hornblende , a particularly important amphibole that contains high levels of calcium , sodium and magnesium in addition to iron , occurs in both igneous and metamorphic rocks such as B. amphibolite . Tremolite , actinolite or nephrite , the latter as the most important component of jade , are mainly found in metamorphic rocks.
There are sites worldwide, so the list of sites mentioned must remain incomplete:
Greenbushes / Western Australia in Australia , Brumado / Bahia in Brazil , Bodenmais in Germany , Québec in Canada , Manono in the Democratic Republic of the Congo , fire bridges in Austria , Snarum and Utö in Sweden , Campolungo in Switzerland , Hermanov in the Czech Republic , Dnipropetrovsk Oblast in the Ukraine , New York in the United States .
use
Until the 1970s, among other things, riebeckite (crocidolite, blue asbestos ) was processed into resistant and fireproof insulation and fabrics.
Heinrich Harrer reports when crossing West Papua in 1962 that the Dani in the area around Mulia like to use blue glaucophane for the manufacture of stone axes in addition to the green epidote . Fires were lit in selected places in the quarry and, hours later, rocks were broken off with rubble stones, wedges and poles and brought to safety with wooden tongs. Harrer is amazed at how quickly the stones are hewn and the rough shape of the stone ax emerges.
The amphibole group gives its name to Amphibole Peak , a mountain in Antarctica.
Precautions
Actinolite, anthophyllite, tremolite and Riebeckite from the amphibole group, like all asbestos known lung diseases such as asbestosis or mesothelioma trigger.
See also
literature
- Bernhard E. Leake, Alan R. Woolley, Charles ES Arps and others: Nomenclature of amphiboles: report of the Subcommittee on Amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names . In: The Canadian Mineralogist . tape 35 , 1997, pp. 219–246 (English, mineralogicalassociation.ca [PDF; 1.4 MB ; accessed on August 18, 2019]).
- Bernhard E. Leake, Alan R. Woolley, William D. Birch and others: Nomenclature of amphiboles: Additions and revisions to the International Mineralogical Association's amphibole nomenclature . In: American Mineralogist . tape 89 , 2004, p. 883–887 (English, minsocam.org [PDF; 188 kB ; accessed on August 18, 2018]).
- Ernst AJ Burke, Bernhard E. Leake: Named Amphiboles: A new category of amphiboles recognized by the international mineralogical association (IMA), and the proper order of prefixes to be used in amphibole names . In: The Canadian Mineralogist . tape 42 , 2004, p. 1881–1883 (English, pubsites.uws.edu.au [PDF; 43 kB ; accessed on August 18, 2018]).
- Roberta Oberti, Massimo Boiocchi, David C. Smith, Olaf Medenbach, Henk Helmers: Potassic-aluminotaramite from Sierra de los Filabres, Spain . In: European Journal of Mineralogy . tape 20 , no. 6 , 2008, p. 1005-1010 , doi : 10.1127 / 0935-1221 / 2008 / 0020-1837 (English).
- Frank C. Hawthorne, Roberta Oberti, George E. Harlow, Walter V. Maresch, Robert F. Martin, John C. Schumacher, Mark D. Welch: IMA Report. Nomenclature of the amphibole supergroup . In: American Mineralogist . tape 97 , 2012, p. 2031–2048 (English, minsocam.org [PDF; 4.9 MB ; accessed on August 18, 2019]).
Web links
- Mineral Atlas: Mineral Group / Amphibole (Wiki)
- Detailed description of the amphiboles on ruby.chemie.uni-freiburg.de