Sorbitol dehydrogenase
| Sorbitol dehydrogenase | ||
|---|---|---|
|
||
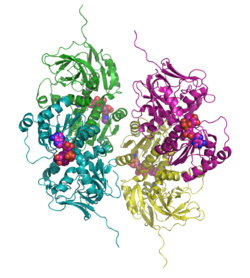
| Ribbon model of the tetramer, NAD and zinc as domes, according to PDB 1PL8 | ||
| Properties of human protein | ||
| Mass / length primary structure | 356 amino acids | |
| Secondary to quaternary structure | Homotetramer | |
| Cofactor | Zinc, NAD | |
| Identifier | ||
| Gene name | SORD | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.1.1.14 , oxidoreductase | |
| Response type | oxidation | |
| Substrate | Sorbitol + NAD + | |
| Products | Fructose + NADH | |
| Occurrence | ||
| Parent taxon | Creature | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 6652 | 20322 |
| Ensemble | ENSG00000140263 | ENSMUSG00000027227 |
| UniProt | Q00796 | Q64442 |
| Refseq (mRNA) | NM_003104 | NM_146126 |
| Refseq (protein) | NP_003095 | NP_666238 |
| Gene locus | Chr 15: 45.02 - 45.08 Mb | Chr 2: 122.23 - 122.27 Mb |
| PubMed search | 6652 |
20322
|
Sorbitol dehydrogenase (Sdh) ( Gen : SORD ) is the name for enzymes that convert sorbitol to fructose . This is the second step in the polyol pathway used by cells to produce fructose from glucose without consuming ATP . Every living being uses Sdh. In mammals, the enzyme is active in all tissue types.
There is evidence that polymorphisms in the SORD gene are associated with reticulopathy in type 2 diabetes mellitus . Sdh inhibitors (or aldose reductase inhibitors) can prevent the occurrence of neurological and ophthalmological problems in diabetes and are therefore of interest as potential drugs.
Structure and function
Sdh forms a complex tetramer that is given its special shape by a network of hydrogen bonds . This mechanism has been preserved in all mammals and is essential for the function of the enzyme.
The catalyzed reaction:
 + NAD (P) + ⇔
+ NAD (P) + ⇔
 + NAD (P) H / H +
+ NAD (P) H / H +
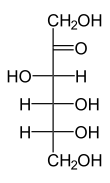
D-sorbitol is dehydrated to D-fructose and vice versa.
Individual evidence
- ↑ El-Kabbani O, Darmanin C, Chung RP: Sorbitol dehydrogenase: structure, function and ligand design . In: Curr. Med. Chem . 11, No. 4, February 2004, pp. 465-76. doi : 10.2174 / 0929867043455927 . PMID 14965227 .
- ↑ UniProt Q00796
- ↑ Szaflik JP, Majsterek I, Kowalski M, et al : Association between sorbitol dehydrogenase gene polymorphisms and type 2 diabetic retinopathy . In: Exp. Eye Res . 86, No. 4, April 2008, pp. 647-52. doi : 10.1016 / j.exer.2008.01.009 . PMID 18289528 .
- ↑ Schmidt RE, Dorsey DA, Beaudet LN, et al : A potent sorbitol dehydrogenase inhibitor exacerbates sympathetic autonomic neuropathy in rats with streptozotocin-induced diabetes . In: Exp. Neurol. . 192, No. 2, April 2005, pp. 407-19. doi : 10.1016 / j.expneurol.2004.12.018 . PMID 15755558 .
- ↑ Kador PF, Inoue J, Blessing K: Anticataract activity of analogs of a sorbitol dehydrogenase inhibitor . In: J Ocul Pharmacol Ther . 20, No. 4, August 2004, pp. 333-44. doi : 10.1089 / 1080768041725281 . PMID 15321028 .
- ↑ Hellgren M, Kaiser C, de Haij S, Norberg A, Höög JO: A hydrogen-bonding network in mammalian sorbitol dehydrogenase stabilizes the tetrameric state and is essential for the catalytic power . In: Cell. Mol. Life Sci. . 64, No. 23, December 2007, pp. 3129-38. doi : 10.1007 / s00018-007-7318-1 . PMID 17952367 .