Thiopurine methyltransferase
| Thiopurine methyltransferase | ||
|---|---|---|
|
||
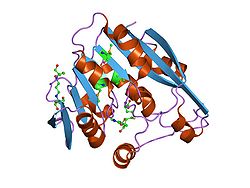
| Belt model of the TPMT according to PDB 2bzg | ||
|
Existing structural data: 1pjz, 2bzg, 2gb4, 2h11, 3bgd, 3bgi |
||
| Properties of human protein | ||
| Mass / length primary structure | 245 amino acids, 35 kD | |
| Secondary to quaternary structure | Monomer | |
| Identifier | ||
| Gene name | TPMT | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 2.1.1.67 , methyltransferase | |
| Response type | Methylation | |
| Substrate | S-adenosylmethionine + thiopurine | |
| Products | S-adenosyl-L-homocysteine + thiopurine-S-methyl ether | |
| Occurrence | ||
| Parent taxon | Bacteria , animals, fungi | |
Thiopurine methyl transferase (Syn. Thiopurine S-methyltransferase, mercaptopurine methyltransferase, 6-thiopurine transmethylase, TPMT ) is an enzyme in the cytosol of animals, fungi and bacteria that methylates thiopurine . This reaction is part of the biotransformation of exogenous substances and a rare deficiency of the enzyme in humans means that drugs containing thiopurines ( 6TG and azathioprine ) act as toxins in normal doses.
Catalyzed reaction
S- adenosyl methionine and thiopurine are converted into S- adenosyl- L- homocysteine and thiopurine- S -methyl ether. The enzyme isinhibitedby S- adenosyl- L- homocysteine.
structure
TPMT is a monomeric protein consisting of a domain with a 9-part core ( β-sheet structure) embedded in 2α-helices. The co-product S-adenosyl-homocysteine (SAH) binds to different areas (α1, α5, α6), as well as to the β-sheet structure 1 and 2. S-adenosyl-homocysteine is completely integrated into the enzyme through the α1-helix which includes the active center .
function
The thiopurine methyl transferase metabolizes azathioprine in the organism . If there is a deficiency in this enzyme, the accumulation of decomposition products of azathioprine can lead to increased side effects.
proof
The thiopurine methyltransferase is determined in a radiochemical assay.
Individual evidence
- ↑ Orthologist at OMA
- ↑ a b UniProt P51580
- ↑ Fujita K, Sasaki Y: Pharmacogenomics in drug-metabolizing enzymes catalyzing anticancer drugs for personalized cancer chemotherapy . In: Curr. Drug metab. . 8, No. 6, August 2007, pp. 554-62. PMID 17691917 .