Ter-sea reaction
The Ter-Meer reaction is a name reaction in organic chemistry and is used to convert a geminal nitrohalogenoalkane into a geminal dinitroalkane. The reaction is named after its discoverer Edmund ter Meer (1852–1931).
Mechanistically, it is an aliphatic nucleophilic substitution of the halide ion ( chloride in the formula example ) by a nitrite ion. For this purpose, a reaction mechanism is proposed in which a proton is split off from the nitroalkane 1 in the first step and the carbanion 2 is formed. This nitrocarbanion is reprotonated to 3 and finally the chloride is nucleophilically substituted by nitrite. The result is the geminal dinitroalkane 4 .
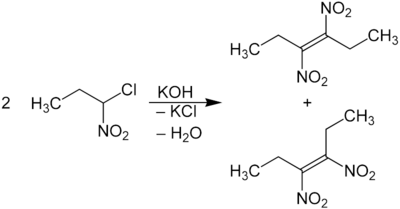
Are nitro haloalkanes contrast with potassium hydroxide treated, a mixture produced in a similar reaction of the cis - trans - isomers [( E ) / ( Z ) isomers] of the vicinal Dinitroalkens:
Individual evidence
- ↑ Edmund ter Meer (1876): About dinitro compounds of the fat series . Justus Liebig's Annalen der Chemie 181 (1): 1–22. doi: 10.1002 / jlac.18761810102 .
- ↑ M. Frederick Hawthorne: aci-Nitroalkanes. I. The Mechanism of the Sea Reaction , J. Am. Chem. Soc. ; 1956; 78 (19), 4980-4984, doi: 10.1021 / ja01600a048 .
- ↑ DE Bisgrove, JF Brown, LB Clapp: 3,4-dinitro-3-hexene in: Organic Syntheses . 37, 1957, p. 23, doi : 10.15227 / orgsyn.037.0023 ; Coll. Vol. 4, 1963, p. 372 ( PDF ).