Thorpe-Ziegler reaction
The Thorpe-Ziegler reaction (also known as the Thorpe-Ziegler ring closure process or just Thorpe cyclization) is a name reaction in organic chemistry and is named after its discoverers, Jocelyn Thorpe and Karl Ziegler . The reaction proceeds analogously to the Dieckmann condensation , but the starting materials used are not dicarboxylic acid esters, but α, ω- dinitriles . This reaction has many applications in the preparation of heterocycles and macrocyclic ketones. In the Thorpe-Ziegler reaction , an α, ω-dinitrile reacts with a sterically hindered base to form a cyclic α-ketonitrile. Acid hydrolysis of the α-ketonitrile produces an α-keto carboxylic acid which, with decarboxylation, produces a cyclic ketone .
If two CH-acidic nitriles react intramolecularly analogously with one another, the Thorpe reaction takes place; this reaction can be regarded as a variant of the Thorpe-Ziegler reaction.
mechanism
Evidence of the possible reaction mechanism was provided by UV spectra, which could be used to identify the intermediate products that were formed.
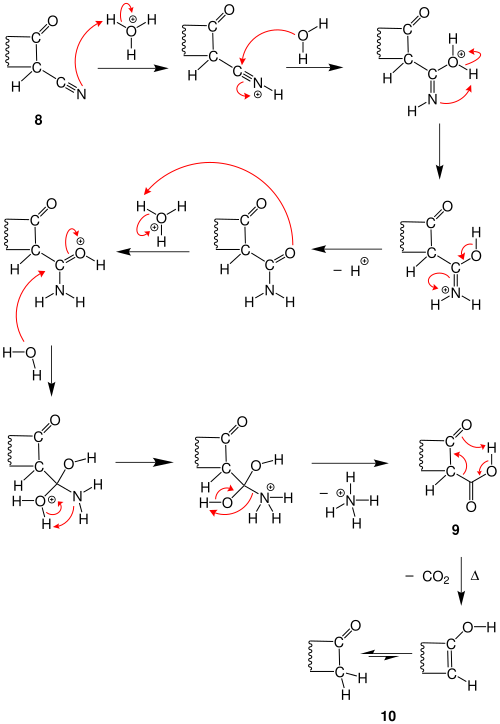
The meandering line in the molecule is supposed to mean a carbon chain of variable length. A nitrile group is attached to each end of this chain , so it is an α, ω-dinitrile 1 . If this molecule is treated with a sterically hindered base (here potassium tert -butanolate ), one of the carbon atoms to which the nitrile group is attached is deprotonated , and at the same time the tert -butanolate reacts to form tert- butanol. The deprotonated form of dinitrile 2 is obtained . The carbon atom just deprotonated now attacks the carbon atom of the ω-nitrile group. This creates the cyclic anion 3 . The abstraction of a proton then provides the imine 4 . By adding acid, this nitrogen atom is protonated once more, which creates the iminium ion 5 . A water molecule now attaches to the carbon atom of the iminium ion, which creates an oxonium group in the molecule. In addition, a superimposed pair of electrons of the double bond to the nitrogen atom so that the iminium ion to the amino group is 6 . The nitrogen atom of this amino group now attacks a hydrogen atom of the oxonium group. This changes the oxonium group to the hydroxyl group and the amino group to the ammonium group 7 . The ammonium group now splits off from the molecule and attacks the hydrogen atom of the hydroxyl group , so that a ketone 8 with a nitrile function in the α-position is formed. The reaction can be interrupted at this point, whereby a cyclic ketone with a nitrile group in the α-position to the carbonyl group is obtained.
Often the nitrile function should be split off in the α-position of the ketone 8 . For this purpose, the nitrile group in 8 is hydrolyzed to the corresponding β-ketocarboxylic acid 9 in several steps . Upon heating, 9 decarboxylates through a six-membered transition state to provide the enol form of the cyclic ketone 10 .
The manufacture of 9- to 13-membered rings is difficult due to transannular stresses, and the yields are correspondingly low. When carrying out a Thorpe-Ziegler reaction, the Ziegler-Ruggli dilution principle is always used in very dilute solution. This ensures that the probability of an intermolecular reaction is lower than that of the desired intra- molecular reaction. This is based on the fact that the probability that the ends of the same molecule will find each other is independent of the concentration of the molecules, while the probability that two molecules will find each other decreases with increasing dilution.
An important application of the Thorpe-Ziegler reaction is the synthesis of thiamine . There it is used to represent the pyrimidine content . N '-cyano- N - (2-cyanoethyl) acetamidine reacts to form 4-amino-2-methyl-1,6-dihydro-5-pyrimidine carbonitrile. This is then further converted to the actually desired product, 4-amino-5-aminomethyl-2-methylpyrimidine.
Individual evidence
- ↑ a b B. P. Mundy, MG Ellerd, FG Favaloro: Name Reactions and Reagents in Organic Synthesis. 2nd edition, Wiley-Interscience, Hoboken, NJ 2005, ISBN 978-0-471-22854-7 , p. 646.
- ↑ Michael B. Smith: March's Advanced Organic Chemistry. 7th edition, John Wiley & Sons, Hoboken, New Jersey, 2013, ISBN 978-0-470-46259-1 , p. 1179.
- ↑ A. Edenhofer et al .: Studies on the Thorpe-Ziegler Reaction - A New Synthesis of the Pyrimidine Part of Thiamine. In: Helvetica Chimica Acta , 58 (4), 1975, pp. 1230-1240.