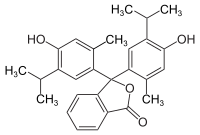
Thymolphthalein
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Thymolphthalein | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 28 H 30 O 4 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 430.55 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
253 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thymolphthalein is a triphenylmethane dye and belongs to the phthalein family. The name is composed of thymol and phthalic anhydride . The corresponding sulfonphthalein is thymol blue . Like phenolphthalein, it is used as a pH indicator .
properties
Thymolphthalein is a white crystalline powder and is practically insoluble in water. It is mostly used in a 0.1% alcoholic solution. It is a weak acid itself.
Dissolved thymolphthalein is colorless at a pH value of 0 to about 9.3, at a higher pH value (10.5) the solution turns blue, in a strongly alkaline medium, at a pH value close to 14, it becomes colorless again . It is therefore u. a. used as an indicator in the titration of basic solutions . It is particularly suitable for determining the second equivalence point of phosphoric acid at pH ≈ 9.7.
presentation
In a Friedel-Crafts acylation , 2 equivalents of thymol and 1 equivalent of phthalic anhydride are reacted in the presence of small amounts of concentrated sulfuric acid or zinc chloride.
Individual evidence
- ↑ a b c d e data sheet thymolphthalein (PDF) from Merck , accessed on December 25, 2019.
- ↑ Jander / Jahr: Maßanalyse , 17th edition, de Gruyter, Berlin 2009, p. 123.

