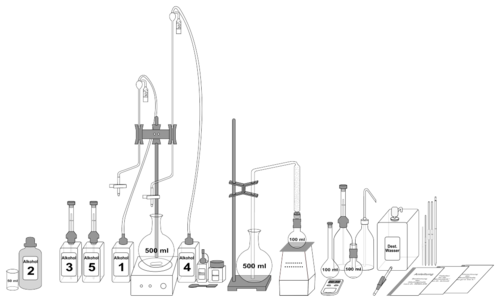
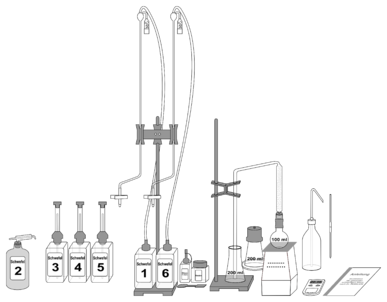
Examination methods according to Dr. Rebelein
The examination methods according to Dr. Rebelein are used to examine drinks for alcohol, sugar and sulfur. They are no longer on the cutting edge of science but are still practiced.
Determination of alcohol content
The alcohol in the beverage sample is distilled over into an acidic potassium chromate solution, with quantitative oxidation to acetic acid taking place. The excess oxidizing agent is then back-titrated with sodium thiosulfate.
Work equipment is a glass apparatus, heating device, magnetic stirrer, laboratory alarm clock and the reagents alcohol 1–5.
| Reagent name | Reagent |
|---|---|
| Alcohol 1 | Potassium chromate solution |
| Alcohol 2 | nitric acid |
| Alcohol 3 | Potassium iodide solution |
| Alcohol 4 | Sodium thiosulphate solution |
| Alcohol 5 | Starch solution |
Determination of the sugar
The reducing sugars are oxidized with an alkaline copper sulfate solution, whereby the divalent copper is reduced to the monovalent copper oxide . After adding potassium iodide , an amount of iodine equivalent to the unused copper sulfate is released, which can be back-titrated with sodium thiosulfate. The inversion of any sucrose is done by boiling with dilute sulfuric acid .
Work equipment and a glass apparatus, heater, laboratory alarm clock and reagents sugar 1–6.
| Reagent name | Reagent |
|---|---|
| Sugar 1 | Copper sulfate solution |
| Sugar 2 | Potassium Sodium Tartrate Solution |
| Sugar 3 | Potassium iodide solution |
| Sugar 4 | Sulfuric acid solution |
| Sugar 5 | Starch solution |
| Sugar 6 | Sodium thiosulphate solution |
Distillation of all the sulphurous acid
For the determination of total sulphurous acid ( distillation ). All of the sulphurous acid is distilled over into an alkaline potassium iodate solution. After the initial charge has been acidified, the excess oxidizing agent is back-titrated with sodium thiosulphate solution.
Working materials are glass apparatus, heating equipment, laboratory alarm clock and reagents sulfur 1–6
| Reagent name | Reagent |
|---|---|
| Sulfur 1 | Potassium iodate solution |
| Sulfur 2 | Methanol |
| Sulfur 3 | Sulfuric acid solution |
| Sulfur 4 | Starch solution |
| Sulfur 5 | Sulfuric acid solution |
| Sulfur 6 | Sodium thiosulphate solution |
Rapid titration of total SO 2 , free SO 2 and total acidity of white wines
To determine total sulfur dioxide (SO 2 ), free SO 2 and total acid ( titration ), a beverage sample is made alkaline to saponify the bound sulfur dioxide, mixed with a defined excess amount of iodate solution, acidified, then mixed with iodide and the excess formed Iodine back-titrated with sodium thiosulfate.
A glass apparatus and the reagents Sulfur 11–55 serve as working equipment.
| Reagent name | Reagent |
|---|---|
| Sulfur 11 | Caustic soda |
| Sulfur 22 | Potassium iodate solution with starch |
| Sulfur 33 | Sulfuric acid solution |
| Sulfur 44 | Starch solution |
| Sulfur 55 | Sodium thiosulphate solution |
literature
- Tanner / Brunner: Beverage Analysis - Verlag Heller Chemie- und Verwaltungsgesellschaft, Schwäbisch Hall 1987, ISBN 978-3-9800498-1-8 .
- Alfred Schmitt: Current wine analysis - Verlag Heller Chemie- und Verwaltungsgesellschaft, Schwäbisch Hall 2005, ISBN 978-3-9800498-3-2 .
Web links
- Determination of alcohol content according to Dr. Rebelein - german pdf (196 kB)
- Determination of alcohol according to Dr. Rebelein - english pdf (393 kB)
- Determination of sugar according to Dr. Rebelein - german pdf (177 kB)
- Determination of sugar according to Dr. Rebelein - english pdf (177 kB)
- Distillation of the entire sulfurous acid according to Dr. Rebelein - german pdf (179 kB)
- Distillation of the total sulphurous acid according to Dr. Rebelein - english pdf (177 kB)
- Rapid titration of total SO 2 , free SO 2 and total acidity of white wines according to Dr. Rebelein - german pdf (915 kB)
- Titration of total SO 2 , free SO 2 and total acid in white wines according to Dr. Rebelein - english pdf (917 kB)