Copper (I) oxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Cu + __ O 2− | |||||||||||||||||||
| Crystal system |
cubic |
||||||||||||||||||
| Space group |
Pn 3 m (No. 224) |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Copper (I) oxide | ||||||||||||||||||
| other names | |||||||||||||||||||
| Ratio formula | Cu 2 O | ||||||||||||||||||
| Brief description |
yellow to red-brown, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 143.09 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
6.0 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
1232 ° C |
||||||||||||||||||
| boiling point |
thermal decomposition around 1800 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.1 mg m −3 (measured as the inhalable aerosol fraction) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Copper (I) oxide is a chemical compound that contains copper and oxygen . In this oxide with the empirical formula Cu 2 O, copper is monovalent . Copper (I) oxide is a yellow to red-brown solid and turns black when heated, but returns to its original color after cooling.
Occurrence
Copper (I) oxide occurs naturally as the mineral cuprite . Cuprite is formed during the weathering of copper sulphides and is therefore usually found in oxidized parts of copper deposits.
Extraction and presentation
Copper (I) oxide can be prepared by the reduction of copper (II) oxide with metallic copper at elevated temperature in a comproportionation reaction or by thermal decomposition of copper (II) oxide at temperatures above 800 ° C can be produced. Copper (I) oxide forms together with copper (II) oxide when metallic copper is heated to red heat in air.
Copper (I) oxide can also be produced by reducing copper (II) salts in an alkaline solution or by reducing copper (II) hydroxide . Suitable reducing agents are, for example, hydrazine and aldehydes . The reduction of copper (II) salts is used in organic chemistry for the detection of reducing sugars with the Fehling sample .
Copper (I) oxide can be produced by the reaction of copper (I) chloride or copper (I) iodide with alkali hydroxides.
properties
Copper (I) oxide is a yellow to red powder, depending on the particle size, and is practically insoluble in water . In contrast, it is soluble in dilute acids . Copper (I) oxide is soluble in ammonia or ammonia water with complex formation . The [Cu (NH 3 ) 2 ] + complex formed is, in contrast to the [Cu (H 2 O) 4 ] + complex, stable. The latter disproportionates into Cu and [Cu (H 2 O) 6 ] 2+ .
Moist copper (I) oxide is easily oxidized to blue copper (II) hydroxide in the air. In contrast, dry copper (I) oxide does not react.
Copper (I) oxide is reduced to metallic copper by hydrogen at an elevated temperature .
Copper (I) oxide reacts with dilute sulfuric acid to form copper (II) sulfate and metallic copper ( disproportionation ).
Copper (I) oxide reacts with dilute nitric acid to form copper (II) nitrate and metallic copper (disproportionation).
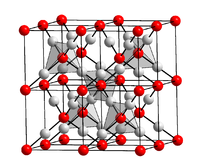
It has a cubic crystal structure with the space group Pn 3 m (space group number 224) , a = 4.268 Å , and an enthalpy of formation of −170.3 kJ / mol.
use
Copper (I) oxide is used for anti-fouling paints, for example underwater paints . The dissolving copper is poisonous for algae and inhibits their build-up on the ship's walls. In addition, copper (I) oxide is used as a starting material for the production of various copper compounds. It is also used as a pigment for coloring glass and enamel red , for producing gold flux , as a fungicide and as a catalyst .
Copper (I) oxide is also a semiconductor with a direct band gap of approx. 2 eV and was used for the construction of rectifier diodes as early as the 1920s . Such copper oxide rectifiers consisted of copper disks coated with Cu 2 O. Selenium and later germanium and silicon later displaced Cu 2 O as a semiconductor material, but basic studies are still being carried out on Cu 2 O, because excitons can be excited very easily in this material .
In November 2019 the University of Waterloo (Canada) presented a process in which copper (I) oxide serves as a catalyst for the "artificial leaf". Here, carbon dioxide (CO 2 ) is introduced into a mixture of water and copper (I) oxide. When exposed to visible light, a chemical reaction takes place in which CO 2 reacts to form oxygen and methane . With this process, renewable methane can be produced economically as an alternative to fossil fuel .
Web links
- Mineralogical data to cuprite (English)
Individual evidence
- ↑ Entry on CUPROUS OXIDE in the CosIng database of the EU Commission, accessed on March 4, 2020.
- ↑ a b c d e f g h i Entry on copper (I) oxide in the GESTIS substance database of the IFA , accessed on October 18, 2018(JavaScript required) .
- ↑ a b Entry on copper oxides. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Entry on Dicopper oxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on May 22, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 979.
- ^ Scientists create 'artificial leaf' that turns carbon dioxide into fuel. University of Waterloo, November 4, 2019, accessed November 10, 2019 .












