Valence isomerism
The valence isomerization or valence tautomerism is a special case of isomerism in organic compounds . The term from stereochemistry describes the difference in the constitution of compounds with the same molecular formula , which results from rearrangement of σ or π bonds .
Examples
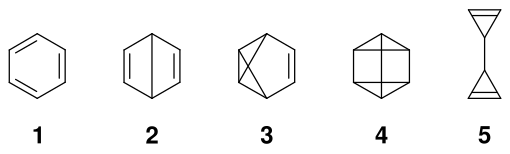
Valence isomers of benzene
For organic compounds with the empirical formula C 6 H 6, there are 5 ways to arrange the six methine groups . In addition to benzene 1 , its valence isomers Dewar-Benzol 2 , Benzvalene 3 , Prisman 4 and Bicyclopropenyl 5 are obtained .
Valence isomers of benzene
Pericyclic reactions
Many valence isomerizations are pericyclic reactions . Important examples are the Cope and Claisen rearrangements . A degenerate valence - even topomerization called - is when starting compound and product can not be distinguished, for example in the Cope rearrangement of 3,4-homotropilidene or barbarian Alan .
The C 10 H 10 hydrocarbon Bullvalen contains three homotropilid structural elements. This results in around 1.2 million possible valence isomers, which are in rapid equilibrium with one another at higher temperatures. In the various valence isomers of bullvalene, each carbon atom is linked to each of the other nine carbon atoms and can occupy any position in the molecule (cyclopropane ring, bridgehead atom, olefinic position adjacent to the cyclopropane ring or adjacent to the bridgehead).
The three Cope systems (red) of a bullvalene molecule, with the respective
valence isomers of the intramolecular Cope rearrangement
Individual evidence
- ^ Entry on valence isomerism. In: Römpp Online . Georg Thieme Verlag, accessed on September 25, 2019.
- ↑ Arthur H. Schmidt: valence isomers of benzene . In: Chemistry in Our Time . tape 11 , no. 4 , August 1977, p. 118 , doi : 10.1002 / ciuz.19770110404 .
- ↑ Entry on valence isomerization. In: Römpp Online . Georg Thieme Verlag, accessed on September 25, 2019.
- ↑ Gerhard Schröder: Synthesis and properties of tricyclo [3.3.2.04.6] decatrien- (2.7.9) 2.3) (Bullvalen) . In: Chemical Reports . tape 97 , no. November 11 , 1964, pp. 3140 , doi : 10.1002 / cber.19640971125 .