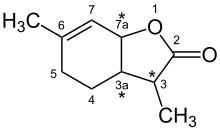
Wine lactone
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Wine lactone | |||||||||
| other names |
(3 S , 3a S , 7a R ) -3,6-dimethyl-3a, 4,5,7a-tetrahydro-3 H -1-benzofuran-2-one |
|||||||||
| Molecular formula | C 10 H 14 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 166.22 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
50-51 ° C (3 S , 3a S , 7a R ) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
The wine lactone is in the wine -occurring chemical compound from the group of bicyclic lactones . It has an intensely sweet and coconut-like odor that can still be perceived even when diluted.
Isomers
Due to the structure of the molecule with three stereocenters, there are four pairs of enantiomers or eight diastereomers .
The actual wine lactone is in the (3 S , 3a S , 7a R ) configuration and has the lowest odor threshold of all isomers , it can already be perceived in a concentration of 0.00001 to 0.00004 nanograms per liter of air.
The very different odor thresholds of the eight isomers in air were determined as follows:
| Structural formula | Configuration according to the CIP convention | CAS number | Odor threshold [ng / l] |
|---|---|---|---|
 |
(3 S , 3a S , 7a S ) | 182699-81-6 | 0.007-0.014 |
 |
(3 R , 3a R , 7a R ) | 14-28 | |
 |
(3 R , 3a R , 7a S ) | 182699-78-1 | > 1000 (odorless) |
 |
(3 R , 3a S , 7a S ) | 182699-83-8 | 8-16 |
 |
(3 S , 3a R , 7a R ) | 0.05-0.2 | |
 |
(3 S , 3a S , 7a R ) | 182699-77-0 | 0.00001-0.00004 |
 |
(3 S , 3a R , 7a S ) | 182699-80-5 | 80-160 |
 |
(3 R , 3a S , 7a R ) | 182699-79-2 | > 1000 (odorless) |
The racemate has the CAS number 78168-36-2. The isomers can be separated by gas chromatography on a chiral stationary phase .
presentation
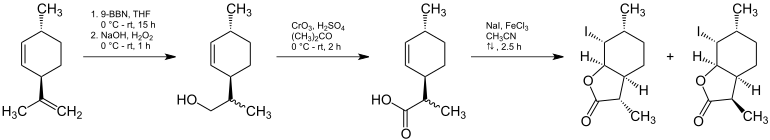
(3 S , 3a S , 7a R ) wine lactone and (3 R , 3a S , 7a R ) wine lactone can be prepared from (1 R ) - trans -isolimones.
After a multi-stage reaction, a mixture of (3 S , 3a S , 6 R , 7 R , 7a R ) -7-iodo-3,6-dimethylhexahydrobenzofuran-2 (3 H ) -one and (3 R , 3a S , 6 R , 7 R , 7a R ) -7-iodo-3,6-dimethylhexahydrobenzofuran-2 (3 H ) -one, which can be separated by chromatography. The desired isomer can then be reacted with diazabicycloundecene to give the corresponding wine lactone.

Individual evidence
- ↑ a b c d Stefano Serra, Claudio Fuganti: Natural p-Menthene Monoterpenes: Synthesis of the Enantiomeric Forms of Wine Lactone, Epi-wine Lactone, Dill Ether, and Epi-dill Ether Starting from a Common Intermediate . In: Helvetica Chimica Acta . tape 87 , no. 8 , August 2004, ISSN 0018-019X , p. 2100–2109 , doi : 10.1002 / hlca.200490189 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ H. Guth: Determination of the configuration of wine lactone. In: Helvetica Chimica Acta. Vol. 79, Issue 6, 1996, pp. 1559-1571; doi: 10.1002 / hlca.19960790606 .
- ↑ a b H.-D. Belitz among other things: Textbook of food chemistry. 5th edition. Springer, Berlin et al. 2001, pp. 343-345. ( limited preview in Google Book search).
- ↑ Subhash P. Chavan, Rajendra K. Kharul, Anil K. Sharma, Sambhaji P. Chavan: An efficient and simple synthesis of ( -) - wine lactone . In: Tetrahedron: Asymmetry . tape 12 , no. 21 , November 2001, ISSN 0957-4166 , p. 2985-2988 , doi : 10.1016 / S0957-4166 (01) 00511-0 .