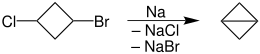Wurtz synthesis
The Wurtz synthesis (or Wurtz reaction ) is a name reaction from organic chemistry , which was discovered in 1854 by the French chemist Adolphe Wurtz (1817-1884). It is used for the synthesis of ( cyclo ) alkanes starting from haloalkanes .
Overview reaction
The reaction is a coupling of two haloalkanes to form symmetrical alkanes.
Sodium is usually used as a reducing agent . The radicals R are alkyl groups .
mechanism
The exact details of the mechanism of the reaction have not yet been clarified. However, there is a currently recognized mechanism that goes as follows:
First, the haloalkane reacts with sodium to form a carbanionic organometallic compound . In the second step, the alkyl radical of the metalated compound acts as a nucleophile and attacks another haloalkane. There is a nucleophilic substitution of the halide.
The reactivity of the haloalkanes decreases in the order of alkyl iodides, alkyl bromides and alkyl chlorides. The driving force behind the Wurtz synthesis is the formation of a sodium halide with a high lattice energy .
use
Due to side reactions such as eliminations or rearrangements , the preparative use of the Wurtz synthesis is severely restricted.
- An example in which the yields are over 90% is the construction of strained systems:
- In the intramolecular synthesis reaction of bi cyclobutane from 1-bromo-3-chlorocyclobutane, the side reactions can largely be pushed into the background.
- Using the Wurtz synthesis, cycloalkanes with a ring size of three to six carbon atoms are accessible:
- Another intramolecular Wurtz reaction of 1, n -dihaloalkanes ( n = 3 - 6) takes place here, which was discovered by Freund in 1882 and Gustavsson in 1887.
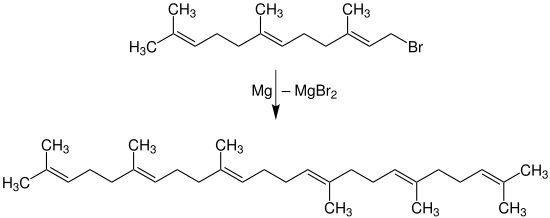
- Squalene is an acylic triterpene hydrocarbon , which was first isolated by Tsyjimoto in 1916 from cod liver oil from sharks. The synthetic production takes place by means of the Wurtz synthesis with magnesium:
- Squalene is u. a. an intermediate stage in the biosynthesis of steroids .
See also
Individual evidence
- ^ A b Siegfried Hauptmann : Organic chemistry. 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 204.
- ^ A b T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 359-360 .
- ^ László Kürti, Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Science & Technology Books, 2005, ISBN 0-12-369483-3 , pp. 498 .
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry. S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 58.
- ^ Siegfried Hauptmann : Organic chemistry. 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 217 f.
- ^ Siegfried Hauptmann: Organic chemistry. 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 695.