Coproporphyrinogen III: Difference between revisions
Jorge Stolfi (talk | contribs) →Biosynthesis and metabolism: one more useful ref |
m Open access bot: doi updated in citation with #oabot. |
||
| (7 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 439935831 |
|||
| ImageFile=Coproporphyrinogen III.svg |
| ImageFile=Coproporphyrinogen III.svg |
||
| ImageSize= |
| ImageSize= |
||
| Line 11: | Line 8: | ||
| CASNo=2624-63-7 |
| CASNo=2624-63-7 |
||
| PubChem = 321 |
| PubChem = 321 |
||
| ChemSpiderID_Ref = {{chemspidercite| |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 315 |
| ChemSpiderID = 315 |
||
| SMILES = Cc1c2[nH]c(c1CCC(=O)O)Cc3c(c(c([nH]3)Cc4c(c(c([nH]4)Cc5c(c(c([nH]5)C2)C)CCC(=O)O)CCC(=O)O)C)CCC(=O)O)C |
| SMILES = Cc1c2[nH]c(c1CCC(=O)O)Cc3c(c(c([nH]3)Cc4c(c(c([nH]4)Cc5c(c(c([nH]5)C2)C)CCC(=O)O)CCC(=O)O)C)CCC(=O)O)C |
||
| Line 18: | Line 15: | ||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChI = 1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48) |
| StdInChI = 1S/C36H44N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h37-40H,5-16H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48) |
||
| StdInChIKey_Ref = {{stdinchicite| |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = NIUVHXTXUXOFEB-UHFFFAOYSA-N |
| StdInChIKey = NIUVHXTXUXOFEB-UHFFFAOYSA-N |
||
| MeSHName=Coproporphyrinogen+III |
| MeSHName=Coproporphyrinogen+III |
||
| Line 43: | Line 40: | ||
==Biosynthesis and metabolism== |
==Biosynthesis and metabolism== |
||
In the main [[porphyrin biosynthesis]] pathway, coproporphyrinogen III is derived from [[uroporphyrinogen III]] by the action of the enzyme [[uroporphyrinogen III decarboxylase]]: |
In the main [[Porphyrin#Synthesis|porphyrin biosynthesis]] pathway, coproporphyrinogen III is derived from [[uroporphyrinogen III]] by the action of the enzyme [[uroporphyrinogen III decarboxylase]]: |
||
[[File: |
[[File:Uroporphyrinogen-decarboxylase.svg|center|480px|alt=Biosynthesis of coproporphyrinogen-III from uroporphyrinogen-III]] |
||
The conversion entails four [[decarboxylation]]s, which turn the four acetic acid groups {{chem2|\sCH2\sCOOH}} into methyl groups {{chem2|\sCH3}}, with release of four [[carbon dioxide]] molecules.<ref name=Hemes>{{cite encyclopedia| |
The conversion entails four [[decarboxylation]]s, which turn the four acetic acid groups {{chem2|\sCH2\sCOOH}} into methyl groups {{chem2|\sCH3}}, with release of four [[carbon dioxide]] molecules.<ref name=Hemes>{{cite encyclopedia|chapter=Hemes in Biology|author=Paul R. Ortiz de Montellano|year=2008|encyclopedia=Wiley Encyclopedia of Chemical Biology|doi=10.1002/9780470048672.wecb221|publisher=John Wiley & Sons|isbn=978-0470048672}}</ref><ref name=sassa>{{cite journal |pmid=10692079|year=2000|last1=Sassa|first1=S.|title=Molecular aspects of the inherited porphyrias|journal=Journal of Internal Medicine|volume=247|issue=2|pages=169–78|last2=Kappas|first2=A.|doi=10.1046/j.1365-2796.2000.00618.x|s2cid=36820694|doi-access=free}}</ref> |
||
Coproporphyrinogen III is further used as a substrate for the enzyme [[coproporphyrinogen III oxidase]] which oxidizes and further decarboxylates it to [[protoporphyrinogen IX]]. |
Coproporphyrinogen III is further used as a substrate for the enzyme [[coproporphyrinogen III oxidase]] which oxidizes and further decarboxylates it to [[protoporphyrinogen IX]]. |
||
| Line 57: | Line 54: | ||
{{DEFAULTSORT:Coproporphyrinogen Iii}} |
{{DEFAULTSORT:Coproporphyrinogen Iii}} |
||
[[Category:Tetrapyrroles]] |
[[Category:Tetrapyrroles]] |
||
{{Biochem-stub}} |
|||
Latest revision as of 14:52, 27 November 2023
| |
| Names | |
|---|---|
| IUPAC name
3-[8,12,17-tris(2-carboxyethyl)-3,7,13,18-tetramethyl-5,10,15,20,21,22, 23,24-octahydroporphyrin-2-yl]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | Coproporphyrinogen+III |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C36H44N4O8 protonated carboxylic acids | |
| Molar mass | 660.757 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
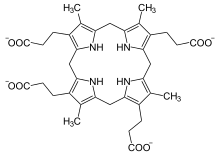
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid.
The compound is a porphyrinogen, a class of compounds characterized by a hexahydroporphine core with various side chains. The coproporphyrinogens have the outermost hydrogen atoms of the core replaced by four methyl groups −CH3 (M) and four propionic acid groups −CH2−CH2−COOH (P). In coproporphyrogen III, the order around the outer ring is MP-MP-MP-PM. For comparison, coproporphyrinogen I has them in the sequence MP-MP-MP-MP. heme.
Biosynthesis and metabolism[edit]
In the main porphyrin biosynthesis pathway, coproporphyrinogen III is derived from uroporphyrinogen III by the action of the enzyme uroporphyrinogen III decarboxylase:

The conversion entails four decarboxylations, which turn the four acetic acid groups −CH2−COOH into methyl groups −CH3, with release of four carbon dioxide molecules.[1][2]
Coproporphyrinogen III is further used as a substrate for the enzyme coproporphyrinogen III oxidase which oxidizes and further decarboxylates it to protoporphyrinogen IX.
References[edit]
- ^ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- ^ Sassa, S.; Kappas, A. (2000). "Molecular aspects of the inherited porphyrias". Journal of Internal Medicine. 247 (2): 169–78. doi:10.1046/j.1365-2796.2000.00618.x. PMID 10692079. S2CID 36820694.
