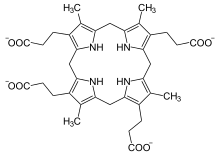
Coproporphyrinogen III
| |
| Names | |
|---|---|
| IUPAC name
3-[8,12,17-tris(2-carboxyethyl)-3,7,13,18-tetramethyl-5,10,15,20,21,22, 23,24-octahydroporphyrin-2-yl]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | Coproporphyrinogen+III |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C36H44N4O8 protonated carboxylic acids | |
| Molar mass | 660.757 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
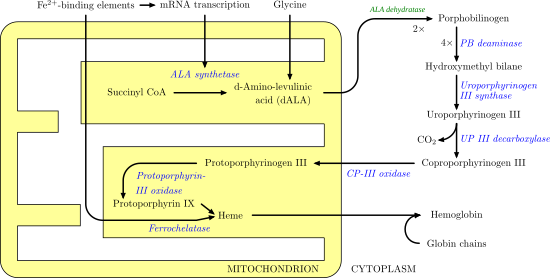
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of heme. It is a colorless compound, classified as a porphyrinogen.
Biosynthetic context
The tetrapyrrole hydroxymethylbilane is converted by the action of uroporphyrinogen-III synthase to uroporphyrinogen III.[1] Uroporphyrinogen III is subsequently converted into coproporphyrinogen III, a conversion that entails four decarboxylations:
- uroporphyrinogen III → coproporphyrinogen III + 4 CO2
Coproporphyrinogen III is further used as a substrate for the enzyme coproporphyrinogen III oxidase which oxidizes and decarboxylates it to protoporphyrinogen IX.
Structural patterns of isomers
The difference between coproporphyrinogen I and III is the arrangements of the four carboxyethyl ("P" groups) and the four methyl groups ("Me" groups). Coproporphyrinogen I has the sequence MeP-MeP-MeP-MeP, whereas in coproporphyrinogen III one MeP-group is reversed and hence an MeP-MeP-MeP-PMe arrangement.
References
- ^ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.