1,3-dihydroxyanthraquinone
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
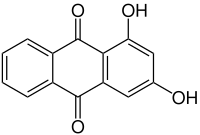
|
|||||||||||||
| General | |||||||||||||
| Surname | 1,3-dihydroxyanthraquinone | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 14 H 8 O 4 | ||||||||||||
| Brief description |
shiny needles of glacial acetic acid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 240.21 g mol −1 | ||||||||||||
| Melting point |
|
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
1,3-Dihydroxyanthraquinone , also known as xanthopurpurine , is a natural substance found in madder . It is an organic compound from the group of anthraquinones (more precisely the dihydroxyanthraquinones ).
Occurrence
Xanthopurpurin, along with other anthraquinones, occurs glycosidically bound in the roots of the madder (lat. Rubia tinctorum ). It also occurs in Indian madder (lat. Rubia cordifolia ).
Presentation and extraction
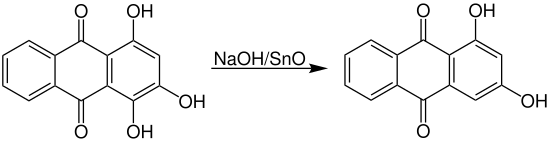
In the past, 1,2,4-trihydroxyanthraquinone ( purpurine ) was treated with tin (II) oxide to replace a hydroxy group .
Today, alkali hydrogen sulfites are more likely to be used, as these are cheaper with higher yields.
It can also be extracted from madder as a natural product.
properties
Xanthopurpurin is very easy to oxidize to purpurin . Since pure, highly diluted purpurin can be discolored by light, one can draw conclusions about the purity of the purpurin and consequently that of the xanthopurpurin.
Individual evidence
- ^ A b Rudolf Bauer, Heinrich Wieland: Reduction and hydrogenation of organic compounds . Springer-Verlag, 1918, ISBN 978-3-662-33872-8 , p. 56 ( limited preview in Google Book search).
- ↑ a b Hans Meyer: Detection and determination of organic compounds , Volume 2, Julius Springer, 1933, ISBN 978-3-662-37854-0 , p. 302.
- ↑ a b H. Plath: About Xanthopurpurin. In: Reports of the German Chemical Society . 9, 1876, pp. 1204-1206, doi : 10.1002 / cber.18760090253 .
- ↑ a b c C. Liebermann, STV Kostanecki: About the coloring properties and the syntheses of oxyanthraquinones. In: Justus Liebig's Annals of Chemistry . 240, 1887, pp. 245-304, doi : 10.1002 / jlac.18872400302 .
- ↑ a b c E. Noah: Synthesis of Xanthopurpurins and Purpurins. In: Reports of the German Chemical Society . 19, 1886, pp. 332-334, doi : 10.1002 / cber.18860190181 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Goverdina CH Derksen, Harm AG Niederländer, Teris A. van Beek: Analysis of anthraquinones in Rubia tinctorum L. by liquid chromatography coupled with diode-array UV and mass spectrometric detection . In: Journal of Chromatography A , 978 (1-2), 2002, pp. 119-127, doi : 10.1016 / S0021-9673 (02) 01412-7 .
- ↑ I. Klöckl: Chemistry of Colorants: In the Painting , Walter de Gruyter GmbH, Berlin 2015, ISBN 978-3-110-37451-3 , p. 291 ( limited preview in the Google book search).