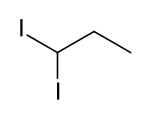
1,1-diiodopropane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | 1,1-diiodopropane | |||||||||
| other names |
1,1-propylidene diiodide |
|||||||||
| Molecular formula | C 3 H 6 I 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 295.8 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
2.48 g cm −3 |
|||||||||
| Melting point |
−49 ° C |
|||||||||
| boiling point |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
1,1-Diiodopropane is a chemical compound that belongs to the halogen alkanes . It is isomeric to 1,2-diiodopropane , 1,3-diiodopropane and 2,2-diiodopropane .
presentation
1,1-Diiodopropane can be made from diazopropane and elemental iodine . The starting material for the production of the diazopropane is propylamine hydrochloride, which is converted into nitrosopropylurea.
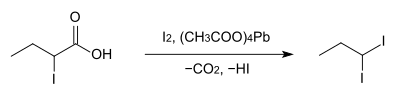
The preparation from 2-iodobutyric acid , iodine and lead (IV) acetate gives poor yields.
properties
The critical temperature of 1,1-diiodopropane is 708.27 K , the critical pressure 42.06 bar . The enthalpy of vaporization at the boiling point is 39.178 kJ / mol.
Individual evidence
- ^ CL Yaws: Thermophysical properties of chemicals and hydrocarbons , 1st edition, p. 112, William Andrew Inc., New York, 2008 . ISBN 0-8155-1596-0 ( limited preview in Google Book Search).
- ^ A b c C. L. Yaws: Thermophysical properties of chemicals and hydrocarbons , 1st edition, p. 8, William Andrew Inc., New York, 2008 . ISBN 0-8155-1596-0 , ( limited preview in Google Book Search).
- ↑ Dictionary of organic compounds, p. 2500 ( limited preview in Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b R. C. Neuman Jr., ML Rahm: Synthesis and Nuclear Magnetic Resonance Spectra of Some 1-Halo-1-iodoalkanes , in: J. Org. Chem. 1966 , 31 , pp. 1857-1859; doi : 10.1021 / jo01344a041 .
- ↑ F. Arndt: Nitrosomethylurea In: Organic Syntheses . 15, 1935, p. 48, doi : 10.15227 / orgsyn.015.0048 ; Coll. Vol. 2, 1943, p. 461 ( PDF ).
- ↑ F. Arndt: Diazomethane In: Organic Syntheses . 15, 1935, p. 3, doi : 10.15227 / orgsyn.015.0003 ; Coll. Vol. 2, 1943, p. 156 ( PDF ).
- ^ Carl L. Yaws: "Thermophysical properties of chemicals and hydrocarbons", 1st edition, p. 315, William Andrew Inc., New York, 2008 . ISBN 0-8155-1596-0 , ( limited preview in Google Book Search).