Dinitrophenols
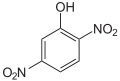
The dinitrophenols ( DNP ) form a group of aromatic compounds that are derived from phenol as well as from nitrobenzene or dinitrobenzenes . The structure consists of a benzene ring with a hydroxyl group (-OH) and two nitro groups (-NO 2 ) as substituents . Their different arrangement results in six constitutional isomers with the empirical formula C 6 H 4 N 2 O 5 , with 2,4-dinitrophenol being the most important. The latter arises from o - and p -nitrophenol by re-nitriding. It is an intermediate product on the way to picric acid .
Dinitrophenols are very toxic and, if contaminated by inhalation, ingestion or contact, cause irritation of the eyes, the digestive tract, blood poisoning, liver damage, dizziness, nausea, headache and irritation of the respiratory tract. 2,4-Dinitrophenol in particular is said to be teratogenic, carcinogenic and mutagenic .
| Dinitrophenols | |||||||||
| Surname | 2,3-dinitrophenol | 2,4-dinitrophenol | 2,5-dinitrophenol | 2,6-dinitrophenol | 3,4-dinitrophenol | 3,5-dinitrophenol | |||
| Structural formula |

|

|
|

|

|

|
|||
| CAS number | 66-56-8 | 51-28-5 | 329-71-5 | 573-56-8 | 577-71-9 | 586-11-8 | |||
| 25550-58-7 (mixture of isomers) | |||||||||
| PubChem | 6191 | 1493 | 9492 | 11312 | 11348 | 11459 | |||
| Molecular formula | C 6 H 4 N 2 O 5 | ||||||||
| Molar mass | 184.11 g mol −1 | ||||||||
| Physical state | firmly | ||||||||
| Brief description | yellow, crystalline solids | ||||||||
| Melting point | 144-146 ° C | 110-112 ° C | 105 ° C | 64 ° C | 132-135 ° C | 122; 126 ° C | |||
| pK s value | 4.09 | 5.04 | 3.71 | 5.42 | |||||
| solubility | soluble in water, soluble in ethanol, ether and chloroform | ||||||||
|
GHS labeling |
|
||||||||
| H and P phrases | 331-311-301-373-410 | ||||||||
| no EUH phrases | |||||||||
|
261-273-280 301 + 310-311 |
261-273-280 301 + 310-311 |
261-273-280 301 + 310-311 |
no P-phrases |
261-273-280 301 + 310-311 |
no P-phrases | ||||
Individual evidence
- ↑ a b Entry for CAS no. 25550-58-7 in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b Entry on 2,3-Dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b Entry on 2,4-dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b Entry on 2,5-dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ^ Entry on 2,6-Dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b Entry on 3,4-Dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c d e CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on 3,5-dinitrophenol in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .



