2-hydroxy-2-methyl-3-oxobutyric acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
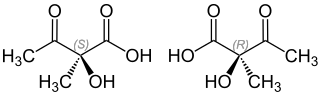
| Structural formula of the ( S ) -form (left) and the ( R ) -form (right) | |||||||||||||
| General | |||||||||||||
| Surname | (2 S ) -2-hydroxy-2-methyl-3-oxobutyric acid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 5 H 8 O 4 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 132.11 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
2-Hydroxy-2-methyl-3-oxobutyric acid is a chiral organic carboxylic acid , the anion of which, 2-acetolactate , also called acetyl lactate , occurs as an intermediate in 2,3-butanediol fermentation , a variant of mixed acid fermentation .
When “2-acetolactate” is mentioned in this text or in the scientific literature without any additional name ( prefix ), it means ( S ) -2-acetolactate (synonym: L -2-acetolactate).
Reaction sequence
2-acetolactate is created by the fusion of two molecules of pyruvate with simultaneous splitting off of one molecule of CO 2 . Then another molecule of CO 2 is split off (by the enzyme 2-acetolactate decarboxylase ) and acetoin is produced , which can be detected with the Voges-Proskauer test . Recently acetoin is NADH / H + reduced and there is 2,3-butanediol .
swell
- Georg Fuchs (ed.): Allgemeine Mikrobiologie , 8th edition, 2007, Thieme Verlag
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
