2-nitrodiphenylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
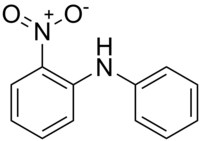
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-nitrodiphenylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 10 N 2 O 2 | ||||||||||||||||||
| Brief description |
orange, crystalline platelets |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 214.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.36 g cm −3 |
||||||||||||||||||
| Melting point |
74-76 ° C |
||||||||||||||||||
| boiling point |
346 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-Nitrodiphenylamine , NDPA for short or 2-NDPA is an orange-red organic compound , a nitrated derivative of the aromatic amine diphenylamine and is isomeric to 3-nitrodiphenylamine and 4-nitrodiphenylamine .
properties
2-Nitrodiphenylamine is strongly polar and has an electrical dipole moment of 4.13 Debye (at 20 ° C in a benzene solution). The orange-colored, crystalline compound still dissolves little in water, but does well in ethanol .
use
2-Nitrodiphenylamine is used as a stabilizer of fuels - such as Otto II fuel - and explosives as well as in the manufacture of synthetic rubber and other polymers. The compound is also used in some synthetic routes in organic chemistry. As a 5 mM solution in dilute sulfuric acid , NDPA is used as a redox indicator for the titrimetric determination of Fe 2+ ions.
Individual evidence
- ↑ a b c d J. IG Cadogan: Dictionary of organic compounds, Volume 5. 6th Edition, CRC Press, 1996, ISBN 978-0-412-54090-5 , p. 3775.
- ↑ a b Entry 2-NITRODIPHENYLAMINE at chemicalland21.com, accessed on December 5, 2016.
- ↑ a b c data sheet 2-Nitrodiphenylamine from Sigma-Aldrich , accessed on December 5, 2016 ( PDF ).
- ^ Carl L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 363 ( limited preview in Google Book search).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 416 ( limited preview in Google Book search).
- ↑ Manfred D. Lechner, J. D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker, Volume 1. 4th edition, Springer, 1992, ISBN 978-3-540-52895-1 , p. 544.
