5,6-diaminobenzo-1,2,3,4-tetracarbonitrile
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 5,6-diaminobenzo-1,2,3,4-tetracarbonitrile | |||||||||
| Molecular formula | C 10 H 4 N 6 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 208.15 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
5,6-Diaminobenzo-1,2,3,4-tetracarbonitrile is an organic molecule with four cyano and two amino functions. The molecule was manufactured in 2016 at the Max Planck Institute for Polymer Research and has the largest measured dipole moment in a neutral molecule to date . The molecule took second place in the Chemical & Engineering News Molecule of the Year election in 2016 .
application
High dipole moments in benzene derivatives are achieved by opposing electron-withdrawing and electron-donating substituents. These uncharged benzene derivatives with a high dipole moment may be suitable as ferroelectric and for applications in nonlinear optics . In addition, such molecules may possibly achieve better charge separation in multilayer solar cells .
Manufacturing
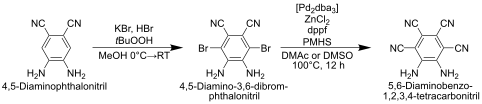
5,6-Diaminobenzo-1,2,3,4-tetracarbonitrile is available via the oxidative bromination of 4,5-diaminophthalonitrile. In the first step, 4,5-diamino-3,6-dibromophthalonitrile is produced, which can be converted into the target molecule under palladium catalysis with zinc cyanide .
A dipole moment of 14.1 ± 0.7 Debye was measured in dimethylacetamide , the high dipole moment being attributed to complex formation with the solvent. It even exceeds the dipole moment of typical salts such as potassium bromide in the gas phase, which has a dipole moment of 10.41 Debye.
Web links
- Katrina Krämer: A small molecule's big moment. In: chemistryworld.com. Chemistry World , February 8, 2016, accessed December 30, 2016 .
literature
- Mark Peplow: The Record Breakers . In: ACS Central Science . tape 2 , no. 8 , 2016, ISSN 2374-7943 , p. 489-492 , doi : 10.1021 / acscentsci.6b00211 .
Individual evidence
- ↑ a b c d Jakob Wudarczyk, George Papamokos, Vasilis Margaritis, Dieter Schollmeyer, Felix Hinkel, Martin Baumgarten, George Floudas, Klaus Müllen: Hexasubstituted Benzenes with Ultrastrong Dipole Moments. In: Angewandte Chemie International Edition. 55, 2016, pp. 3220–3223, doi: 10.1002 / anie.201508249 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Steve Ritter: Molecules of the year, C&EN highlights some of the coolest compounds reported in 2016
- ↑ Lap Tak Cheng, Wilson Tam, Sylvia H. Stevenson, Gerald R. Meredith, Geert Rikken, Seth R. Marder: Experimental investigations of organic molecular nonlinear optical polarizabilities. 1. Methods and results on benzene and stilbene derivatives. In: The Journal of Physical Chemistry. 95, 1991, pp. 10631-10643, doi: 10.1021 / j100179a026 .
- ^ Betty Isabelle Bleaney, Brebis Bleaney: Electricity and Magnetism, Volume 2 Third Edition . OUP Oxford, 2013, ISBN 978-0-19-964543-5 , pp. 303 ( limited preview in Google Book search).