Acetal formation
The acetal (acetalization) is a chemical reaction in which an acetal is formed. It can be done in a number of ways. The formation from aldehydes is particularly well known . An acetal is the condensation product of an aldehyde or ketone and one or two alcohols (“ hemiacetal ” or “full acetal”).
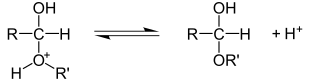
General representation
This reaction is a true equilibrium reaction ; H. the equilibrium can be far to the right or far to the left. The equilibrium position can be changed by manipulating the reaction conditions. The water formed can be continuously removed from the reaction mixture (e.g. by distillation ) or the alcohol is added in excess.
Addition reaction from aldehydes
Aldehydes (alkanals) react with alkanols to form hemiacetals . In an acidic environment, these react further to form acetals when water escapes .
The analogous reaction with ketones produces ketals ; with diols or polyols , cyclic acetals are formed, such as isopropylidene glycerine ( solketal ) from acetone and glycerine :
Reaction mechanism
Hemiacetal formation
1. Protonation of the carbonyl group of an alkanal or aldehyde:
2. Nucleophilic attack by the hydroxy group
3. Release of a proton (recovery of the catalyst):
Acetal formation
1. Protonation of the hydroxy group of the hemiacetal:
2. Elimination of water (H 2 O):
3. Nucleophilic attack
4. Elimination of a proton :
Addition reaction from ketones
Acetals can also be formed from ketones ( alkanones ), although this reaction is slower than with aldehydes or alkanals. The ketal formation proceeds analogously to the acetal formation.
Acetals as protecting groups
When an aldehyde or ketone is converted into an acetal, the reactive carbonyl group is converted into a relatively inert, ether-like unit. Since the acetal is reversible, makes you look so in syntheses to Use to carbonyl groups before conversions protect .
literature
- Jonathan Clayden, Nick Greeves, and Stuart G. Warren: Organic Chemistry. 2nd Edition. Springer, Berlin 2013, ISBN 978-3-642-34715-3 .