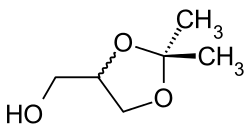
Isopropylidene glycerine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Isopropylidene glycerine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 O 3 | ||||||||||||||||||
| Brief description |
colorless liquid (racemate) |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 132.16 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.063 g / ml (racemate) |
||||||||||||||||||
| Melting point |
−26.4 ° C |
||||||||||||||||||
| boiling point |
188-189 ° C (racemate) |
||||||||||||||||||
| Vapor pressure |
450 mbar (50 ° C) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| Refractive index |
1.434 (20 ° C) (racemate) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Isopropylidene glycerine , also known as Solketal , is a chemical compound from the group of acetonides and dioxolanes . The derivative of glycerol with an isopropylidene protective group is liquid at room temperature.
Manufacturing
Solketal is made synthetically from glycerine and acetone in the presence of acids .
Stereochemistry
Isopropylidene glycerol has a stereocenter and there are therefore two different and mutually enantiomeric compounds ( R ) - and ( S ) -Solketal.
Chemical properties
The isopropylidene acetal group is a so-called protective group for two hydroxyl groups , which means that a selective reaction can take place on the third, still free hydroxyl group. Later you can split off the protective group in the acid and get the two hydroxyl groups back.
use
Isopropylidene glycerine is used as a synthesis component in the chemical and pharmaceutical industries.
Individual evidence
- ↑ Entry on ISOPROPYLIDENEGLYCEROL in the CosIng database of the EU Commission, accessed on June 19, 2020.
- ↑ a b c d data sheet DL-1,2-isopropylideneglycerol, ≥98.0% (GC) from Sigma-Aldrich , accessed on January 16, 2013 ( PDF ).
- ↑ a b c d e f Entry on 2,2-dimethyl-4-hydroxymethyl-1,3-dioxolane in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Mary Renoll, Melvin S. Newman: dl-Isopropylideneglycerol : In Organic Synthesis . 28, 1948, p. 73, doi : 10.15227 / orgsyn.028.0073 ; Coll. Vol. 3, 1955, p. 502 ( PDF ).


