Alkali philia
Alkali philia is the property of living beings to prefer an alkaline environment (i.e. with a high pH value ). Living beings with this property are called alkaliphilic . The linguistically incorrect form of alkalophilicity (or alkalophil ) is often used.
Organisms that are adapted to a medium pH are called neutrophils . Acidophilic organisms are adapted to very acidic environmental conditions with a low pH value.
Alkaliphilic microorganisms
Alkaliphilic organisms mostly occur in highly basic biotopes such as B. carbonate soils and alkaline soda lakes . The alkaliphilic microorganisms include, for example, cyanobacteria of the genus Spirulina and Arthrospira platensis . Extremely alkaliphilic bacteria belong in particular to the genera Bacillus and Clostridium .
Some alkaliphilic bacteria are able to change their environment by alkalizing a neutral medium or acidifying a highly alkaline medium, thereby optimizing the pH value for their growth. Your extracellularly excreted enzymes have their optimum in the strongly alkaline range. Such enzymes have e.g. B. in the detergent industry an important technical importance.
Alkaliphilic microorganisms are extremophilic : They belong to those living things that are adapted to an extreme habitat. The optimum pH of growth is pH 10. Some can grow in strongly alkaline media with a pH of 11.
Maintaining a neutral pH value inside the cell
Their adaptation to alkaline habitats requires overcoming fundamental problems. A high pH level inside the cells is destructive. For example, DNA and especially RNA are hydrolytically decomposed at high pH values . For protection, alkaliphilic organisms need a mechanism with which they limit the increase in the pH value inside the cell, which is threatened by their alkaline environment.
Fig. 1 shows the effect of the increasing alkalinity of the nutrient medium to the right on the pH value inside the cell. In the range up to about pH 10.5, the alkaliphilic bacteria shown there in blue show hardly any higher pH values inside than the two neutrophilic reference organisms shown in green. In the case of the bacterium Clostridium paradoxum , for example, it has been shown that the pH value inside becomes increasingly unfavorable physiologically when the alkalinity rises above this value.
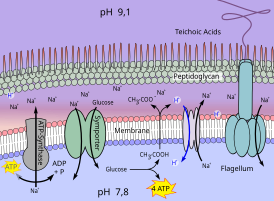
The mechanisms for adapting Clostridium paradoxum to the alkaline environment are shown in Fig. 2 .
Clostridium paradoxum is a thermophilic alkaliphilic anaerobic bacterium that can obtain ATP through homoacetate fermentation of glucose , among other things . While consuming ATP, the organism can use an ATP synthase to transport Na + through the membrane. In addition, the acetogenic bacterium has a ferredoxin in its cell membrane: NAD oxidoreductase ( Rnf complex ), which functions as a Na + ion pump.
Both result in a chemiosmotic membrane potential ΔP, which is composed of the electrical voltage ΔΨ (positively charged on the outside) and the ΔNa + concentration difference . Back-flowing Na + ions enable the import of glucose. Its fermentation supplies acetic acid, which diffuses through the membrane to the outside. In the alkaline exterior, acetic acid dissociates into the acetate anion and H + . By a Na + / H + antiporter the H reaches + back into the cell and acts contrary to the alkalization. The bacterium uses the acid produced during fermentation to stabilize its internal pH value.
The cell wall of alkaliphilic bacteria is specially enriched with teichonic acids, the negative charge of which forms a barrier against the OH - ions in the medium. The space between the cell wall made of peptidoglycan and the cell membrane can thus have a significantly lower pH value than the alkaline medium in which the bacterium lives. A pH gradient occurs within this space . The pH value is lowest directly on the outside of the cell membrane.
Chemiosmosis of alkaliphilic bacteria
The energy metabolism of almost all organisms is based on the operation of ATP synthases . It enables a significantly higher ATP yield than substrate chain phosphorylation in fermentation processes to which comparatively few chemotrophic anaerobic organisms are limited.
The chemiosmotic energy for the endergonic ATP formation catalyzed by the ATP synthase is provided by the vast majority of organisms by building up an electrical voltage and an energy-rich proton gradient on their cell membrane through H + export . The reverse current of the H + ions through the ATP synthase provides this enzyme with energy.
Alkaliphilic organisms cannot build up a membrane potential by releasing H + ions into their nutrient medium. These cations would react immediately with the OH - anions to form water, the energy would be released as heat and would no longer be usable for the organisms. For a long time it was assumed that alkaliphilic organisms must use a Na + -driven ATP synthase to generate energy. This assumption was reinforced by the fact that Na + -ATP synthases were found in Clostridia such as Clostridium paradoxum . Such Na + driven ATP synthases are not typical for alkaliphilic organisms, but for acetogenic bacteria , which also include Clostridium paradoxum , and for methanogenic archaea . These two groups are by no means restricted to alkaline biotopes .
In the case of some alkaliphilic organisms, biochemical analyzes provided evidence of H + driven ATP synthases. When the genomes of a number of different alkaliphilic organisms were compared with those of their close neutrophil relatives, genes for ATP synthases were regularly found that did not show any significant differences. Mutants with reduced ability to grow at high pH values provided a decisive indication of the material basis of alkali philia. They showed deficits in the structure of their cell wall and the space between the cell wall and the cell membrane.
The molecular basis of the alkaliphilia of the alkaliphilic bacterium Bacillus pseudofirmus is shown schematically in Fig. 3. B. pseudofirmus grows aerobically on organic media and, as shown in Fig. 1, keeps its cytosol in the neutral range up to a pH of almost 11.
The respiratory chain of this bacterium transports H + through the cell membrane. Various carotenoids and cytochromes are deposited here. The cytochromes also play the role of a proton transporter. They are increasingly formed by Bacillus pseudofirmus at high pH values and low oxygen concentrations. In a certain sense, a channeled current of H + ions to the ATP synthase flows through the molecules attached to the outside of the membrane .
A Mrp antiporter is also supplied with protons. He exchanges H + for Na + . This exchange brings essential advantages to the organism. On the one hand, the re-import of the H + cations counteracts the alkalization of the cells. The Na + ions pumped outwards maintain the electrical voltage on the cell membrane without running the risk of being neutralized by OH - . Inflowing Na + uses the bacterium energetically to absorb nutrients by a symporter and to operate the flagellum. A Na + membrane channel (NaBP) can be opened in the cell if there is a lack of sodium and is opened or closed as overvoltage protection depending on the voltage.
Alkaliphilic cyanobacteria have a fundamentally different mechanism with which they can use an H + membrane potential. Spirulina platensis maintains a pH of 8.2 in the cytoplasm at an external pH of 10. ATP is formed on a thylakoid membrane, which is located in the middle of the cell without contact with the outside world. The medium in this vesicle is even slightly acidic at pH 6.4. As with Bacillus pseudofirmus, an Na + potential is built up on the outside and acidification of the inside of the cell is counteracted with an H + / Na + antiporter.
Individual evidence
- ^ DB Hicks, J. Liu, M. Fujisawa, TA Krulwich: F1F0-ATP synthases of alkaliphilic bacteria: lessons from their adaptations. In: Biochimica et Biophysica Acta . Volume 1797, number 8, August 2010, pp. 1362-1377, doi: 10.1016 / j.bbabio.2010.02.028 , PMID 20193659 , PMC 2890045 (free full text) (review).
- ↑ D. Pogoryelov, J. Yu, T. Meier, J. Vonck, P. Dimroth, DJ Muller: The c15 ring of the Spirulina platensis F-ATP synthase: F1 / F0 symmetry mismatch is not obligatory. In: EMBO reports. Volume 6, number 11, November 2005, pp. 1040-1044, doi: 10.1038 / sj.embor.7400517 , PMID 16170308 , PMC 1371026 (free full text).
- ↑ GM Cook: The Intracellular pH of Clostridium paradoxum, an Anaerobic, Alkaliphilic, and Thermophilic Bacterium. In: Applied and Environmental Microbiology. Vol. 62, No. 12, 1996, pp. 4576-4579.
- ↑ Terry Ann Krulwich: Adaptive Mechanisms of Extreme Alkaliphiles. In: Koki Horikoshi: Extremophiles Handbook. New York 2011, ISBN 978-4-431-53897-4 , p. 123.
- ↑ Youhong Li, Linda Mandelco, Juergen Wiegel: Isolation and Characterization of a Moderately Thermophilic Anaerobic Alkaliphile, Clostridium paradoxum sp. nov. ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. In: Int J Syst Evol Microbiol. 43, July 1993, pp. 450-460. doi: 10.1099 / 00207713-43-3-450
- ↑ Scott A. Ferguson et al: Biochemical and Molecular Characterization of a Na + -Translocating F 1 F o -ATPase from the Thermoalkaliphilic Bacterium Clostridium paradoxum. In: J. Bac. 188, 14, 2006.
- ↑ Gregory M. Cook et al: The Intracellular pH of Clostridium paradoxum, an Anaerobic, Alkaliphilic, and Thermophilic Bacterium. In: Appl. Environ. Microbiol. December 1996 vol. 62 no. 12, pp. 4576-4579.
- ↑ Thomas Meier, Scott A Ferguson, Gregory M Cook, Peter Dimroth, Janet Vonck: Structural Investigations of the Membrane-Embedded Rotor Ring of the F-ATPase from Clostridium paradoxum . In: Journal of Bacteriology . 188, No. 22, 2006, pp. 7759-7764. doi : 10.1128 / JB.00934-06 . PMC 1636304 (free full text).
- ↑ T. Matsuno, I. Yumoto: Bioenergetics and the Role of Soluble Cytochromes for Alkaline Adaptation in Gram-Negative Alkaliphilic. In: BioMed Research International. 2015, p. 1, doi: 10.1155 / 2015/847945 .
- ↑ Laura Preiss et al: Alkaliphilic bacteria with impact on industrial applications, concepts of early life forms, and bioenergetics of ATP synthesis. In: Front. Bioeng. Biotechnol. June 3, 2015. doi: 10.3389 / fbioe.2015.00075
- ↑ Koki Horikoshi (Ed.): Extremophiles Handbook. New York 2011, ISBN 978-4-431-53897-4 .
- ↑ Terry Ann Krulwich: Adaptive Mechanisms of Extreme Alkaliphiles. In: Koki Horikoshi: Extremophiles Handbook. New York 2011, ISBN 978-4-431-53897-4 , pp. 121-122.