Allyl tension
The allyl tension (actually 1,3-allyl tension or English 1,3-allyl strain ) is a term from organic chemistry and was defined by Francis Johnson in 1968 . This is understood to mean the interaction between the 1- and 3- substituents (R 1 and R 3 ) of an allyl system. The 1,2-allyl tension that also exists, however, is of minor importance.
description
An allyl system is a reactive functional group in an organic molecule . It is characterized by a double bond between two carbon atoms with a subsequent single bond to another carbon atom. The simplest molecule with such a group is propene (C 3 H 6 ). The methyl group that is adjacent to the double bond can rotate freely in this molecule, which means that it makes no difference to the energy content of the molecule how the methyl group is spatially arranged. There are no different conformations, only the rotation barrier of 12.6 kJ / mol measured for the rotation of the hydrogen atoms (see also conformer ).
However, if the carbon atom adjacent to the double bond bears various substituents of different sizes instead of hydrogen atoms , the most stable conformation is that in which the smallest substituent lies in the double bond plane. This is because this substituent interacts with the π orbitals of the neighboring double bond, which increases the energy content of the molecule. This increased energy is known as allyl tension . Because every molecule strives for a state with the lowest possible energy content, the single bond between the carbon atoms in the molecule now rotates in such a way that the substituent with which the allyl tension is lowest enters into this interaction. It is therefore a steric hindrance in ( Z ) -alkenes . Electronic effects such as hyperconjugation can also occur. In this case, the sterically smallest residue can also be rotated by ± 30 ° from the plane of the double bond so that the σ bond of the substituent can interact with the HOMO or LUMO of the π bond.
Using the example of very simple allyl systems, calculations resulted in the following relative energy differences :
The 1,3-allyl strain is comparable to the 1,3-diaxial interaction on cyclic aliphatics and the Felkin-Anh rules for carbonyl systems .
Effects
This effect can be used in stereoselective synthesis .
In hydroboration , for example , in which a new substituent is added to the double bond , this is added from the side on whose side the smaller substituent on the vinyl carbon is also located.
If one of the radicals R 3 or R 4 has a functionality that has a directing effect on a reagent that attacks the C = C bond, then one speaks of an active volume , since in a reaction the attack from this volume (from this side ) is preferred, through coordination to the functionality, and thereby a stereochemical information is transferred from the 1-position to the 3-position.
If one of the radicals R 3 or R 4 does not have a coordinating functionality, but rather has a shielding effect due to its steric demands on a reagent that attacks the C = C bond, one speaks of an inert volume because the attack occurs in a reaction is disadvantaged from this volume (from this side). As a result, stereo information is transferred from the 1 position to the 3 position.
Examples
The more voluminous residues increase the steric demand and the diastereoselectivity increases, since the attack can only take place from the front (beta side).
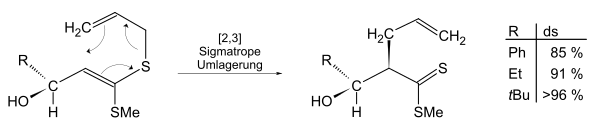
- Inert volume using the example of a [3,3] sigmatropic rearrangement.
meta -chloroperbenzoic acid (mCPBA) iscoordinatedby the benzyl-protected alcohol and the free alcohol and attacks the molecule selectively from behind (alpha side) to form the epoxide .
Further examples of stereoselective syntheses with the aid of allyl tension are cycloadditions , iodolactonizations and epoxidations .
literature
- RW Hoffmann: Allylic 1,3-strain as a controlling factor in stereoselective transformations . In: Chemical Reviews . tape 89 , no. 8 December 1989, pp. 1841–1860 , doi : 10.1021 / cr00098a009 (free full text).
- Lutz F. Tietze, Gerhard Schulz: Ab Initio Molecular Orbital Calculations on Allylic 1,3-Strain of Electron-Donor- and Electron-Acceptor-Substituted Alkenes . In: Liebig's annals . tape 1996 , no. 10 , October 1996, p. 1575-1579 , doi : 10.1002 / jlac.199619961012 .
Individual evidence
- ↑ Francis Johnson: allylic strain in six-membered rings . In: Chemical Reviews . tape 68 , no. 4 , August 1968, p. 375-413 , doi : 10.1021 / cr60254a001 (free full text).
- ↑ a b ( page no longer available , search in web archives: Theoretical consideration of 1,3-allyl voltage (OC-Chemie Uni-Regensburg) ) (PDF; 357 kB).
- ↑ ( Page no longer available , search in web archives: Allyl tension examples ) (PDF; 115 kB).
- ↑ ( page no longer available , search in web archives: application examples in synthesis ) (PDF; 192 kB).



