Artificial sphincter
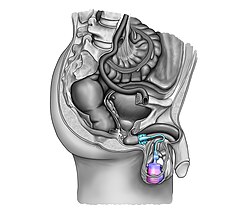
The artificial sphincter ("artificial sphincter", other names: artificial bladder sphincter, artificial urinary sphincter, artificial urethral sphincter, artificial urinary bladder sphincter, urinary bladder sphincter prosthesis ) is a method for the treatment of urinary incontinence in men and women. A cuff that can be inflated via a hydraulic mechanism is placed around the urethra in such a way that it can largely take over the function of the urethral muscle (bladder sphincter). The abbreviation "AUS", which is commonly used in English, is derived from the English name artificial urinary sphincter .
description
There are two types of artificial urinary bladder sphincters to which the two systems on the market correspond:
- The artificial urinary bladder sphincter with a balloon reservoir (3 components): cuff, pump and balloon. The cuff is placed around the urethra ; The pump is implanted in the scrotum and the balloon reservoir is implanted in the retropubic space - between the bladder and pubic bone. The pressure in the hydraulic circuit is generated by the elastic balloon reservoir and from the retropubic pressure.
- The artificial urinary bladder sphincter with a spring (2-component): cuff and pump unit. The cuff is placed around the urethra and the pump unit is implanted in the scrotum. The pressure in the hydraulic circuit is generated by the spring of the pump unit. The pressure in the retropubic space has - for this type of sphincter - no influence.
The common function of the designs currently available is a mechanical locking mechanism with an inflatable cuff filled with sterile saline and placed around the urethra, holding the urethral lumen closed. The urethral cuff is emptied manually by pressing the control pump , which in men is located between two layers of skin of the scrotum (subdartos space), so that the patient can empty the bladder. The urethral cuff is then automatically refilled to close the urethral cuff again to prevent urine from leaking again.
history
Frederic Foley was the first to describe an externally worn artificial urinary bladder sphincter for the treatment of urinary incontinence, published in 1947. In 1972, F. Brantley Scott and colleagues at Baylor College of Medicine designed the first forerunner of the contemporary artificial urinary sphincter. The first AUS model on the market was the AMS 800 ( Boston Scientific , Marlborough , MA ) developed 50 years ago . It is a 3-component device with a cuff around the urethra, a pump inserted in the scrotum and a pressure-generating reservoir located in the pelvis, which is supplied as a kit for preparation and filling prior to implantation.
Another AUS model is the ZSI 375 (Zephyr Surgical Implants, Geneva , Switzerland ) introduced in 2008 . It is a two-part solid construction with a cuff and a pump unit with an integrated spring. It comes as a one-piece device, pre-connected and pre-filled. The ZSI 375 does not contain an abdominal component, which together with its ready-to-implant configuration shortens the implantation time. Since there is no abdominal component, surgical intervention in the retroperitoneal space is not required. Previous operations, such as radical prostatectomy, can lead to postoperative scars and fibrosis in the retroperitoneal space. By avoiding the dissection of retroperitoneal tissue, the risk of surgical complications is avoided. Another benefit of the ZSI 375 model is the ability to increase or decrease the pressure inside the device from the outside after implantation to achieve the desired continence rate and patient satisfaction. These adjustments are particularly helpful in controlling continence in case of urethral atrophy or urinary retention after implantation. The pressure setting can be done on an outpatient basis by adding or removing sterile saline solution through the scrotum via a syringe. By 2019, more than 4,500 ZSI 375 artificial urinary sphincters had been implanted worldwide.
The list includes sphincter models available in 2020:
| product | Companies | Country of origin | Introduced in | design | Pre-connected and pre-filled | Deliver pressure | Adjustable pressure |
|---|---|---|---|---|---|---|---|
| AMS 800 | Boston Scientific ( formerly American Medical Systems ) | United States | 1988 | 3 components: cuff, pump, balloon container | No | Flexible storage container in the pool floor | No |
| ZSI 375 | Zephyr Surgical Implants | Switzerland | 2008 | 2 components: cuff, pump unit | Yes | Stainless steel spring inserted in the pump unit in the scrotum | Yes |
Indications
The intrinsic sphincter deficiency that leads to stress incontinence is the most common indication for an AUS implantation. The European Association of Urology recommends AUS implantation for moderate to severe stress incontinence in men. In addition, despite the novel treatment options (slings, urethral injections, stem cell therapy ) , AUS is considered the gold standard for surgical management of both stress incontinence in men and urinary incontinence, which has been developed as a complication of surgery such as prostatectomy , cystectomy and transurethral resection .
Several case reports have been published in the literature on AUS implantation in children for secondary incontinence due to traumatic urethral injury.
There is limited data on the use of AUS in women and not every product on the market is intended for use in women. The European Association of Urology makes limited recommendations on the use of AUS in women and states that the risk of complications is high, although cure is possible. Despite this, AUS has been used as a last resort in the treatment of urinary incontinence in women due to congenital causes and as a result of neurological disorders.
Surgical intervention
The operation on the man
The implantation is usually carried out under general anesthesia . The procedure lasts about 60 minutes on average. After shaving and disinfecting the surgical field, a catheter is first inserted into the urethra. After making an incision in the perineum or at the point where the penis merges into the scrotum, the urethra is exposed. The cuff is placed around the urethra, the control pump is placed in the scrotum and the pressure-regulating balloon is placed in the lower abdomen. The components are connected to one another by hoses. The surgical wound is closed. The artificial sphincter is only activated several weeks after this operation after complete, also internal wound healing. That is how long the patient remains incontinent.
The operation on the woman
This procedure, known since 1972, is also a possible option for women. In the case of severe stress urinary incontinence in men, it is considered the standard of surgical procedure.
Aftercare
Sexual intercourse should be avoided for the first 6 weeks after the procedure to allow the wound to heal properly. Physical activities that put pressure directly on the wound, such as horse riding and cycling, should also be avoided for at least 6 weeks. Patients can be prescribed a jockstrap to be worn for 1 week after the procedure.
Ongoing maintenance
To minimize the risk of damage to the AUS or urethra , it is important that the patient informs their doctor that an AUS is implanted before a urinary catheter is placed , a cystoscopy, or any other medical procedure is performed on the urinary tract. Disabling the device at night may be recommended to patients, especially those who report being dry at night, to minimize the risk of urethral atrophy.
Results
Success rate
Numerous studies have been published on the results of patients who have had an artificial sphincter implantation. The success rate, which is generally defined as achieving total continence (no use of pads) or social continence (use of ≤ 1 pad / day) with the implanted device, is between 61% and 100% in the literature. The improvement in quality of life was rated as a success even if more than 1 pad / day was required. The success rate was 78% with a follow-up period of 3 years and over 72% with a follow-up period of 5 to 7 years. A recent systematic review reported a success rate of 79% with a follow-up period of 5 months to 16 years. A comparative study among patients implanted with different models of the artificial urinary sphincter who achieved social continence showed no difference between two groups in terms of urodynamic tests such as flow rate , urethral pressure , etc.
satisfaction
In various studies with a mean follow-up time of more than 6 years, at least 73% of men with an implanted artificial urinary sphincter were satisfied or very satisfied with the device, and 10–23% reported dissatisfaction. In shorter follow-up periods (2–4 years) the satisfaction rates reached over 90%. In another study with a mean follow-up time of over 7 years, the overall satisfaction rate was 3.9 on a scale from 0 to 5. The satisfaction rate in patients after radiation therapy does not seem to be negatively influenced. It is reported that initial continence rate satisfaction is improved by adjusting the pressure in the implant with the ZSI 375 model.
Surveys of patients who have had the procedure have found that over 90% recommend the procedure to a friend or relative with the same problem, and over 90% will be reimplanted. At the same time, 14% of patients reported an improvement in sexual activity.
Numerous studies with different scaling parameters have shown that the quality of life improves significantly after the AUS implantation. And quality of life does not seem to be affected by multiple interventions, provided the device continues to work after the overhaul.
Complications
Potential risks arising from the implantation of the AUS are:
- Injury to urethra or bladder during AUS placement;
- Difficulty emptying the bladder requiring temporary self-catheterization;
- prolonged stress urinary incontinence;
- Infection of the device leading to removal;
- Recurrent incontinence due to device failure or atrophy of the urethral tissue (in this case, the * old device can be removed and replaced with a new one during another operation).
The overall reported complication rate in men is 37%. The most common post-operative complications are:
- mechanical failure (8–21%)
- Urethral erosion (4–15%)
- Infection (1–14%)
- Urethral atrophy (4–10%)
Other less common complications are hematoma , urethral stenosis, urinary fistula. Mechanical failures and non-mechanical complications can lead to surgical revision in 8–45% and 7–17% of cases, respectively. The overall device implantation rate in men is reported to be 16 to 20%.
One of the causes of mechanical failure is the complications associated with the balloon reservoir. It was reported that 26% of men with an implanted AUS needed re-operation at the 10-year follow-up to regulate the pressure inside the device.
The risk is also stated with up to 50% in the first five years that new interventions will be necessary.
The operation of the control pump also requires sufficient insight and skill on the part of the patient. Under optimal conditions, an average shelf life of five to ten years can be assumed.
In the largest series available to evaluate 1082 patients who received a primary AUS placement, the 5-year survival rate of the devices was 74%, which is in agreement with the results reported in the literature and is between 59% and 79% . In particular, some patients in all series had to undergo repeated surgery over time due to repeated urinary incontinence or infection of the device. In a pooled analysis of the available studies, the re-intervention rate was approximately 26%. Some studies have shown that surgeons who perform this procedure more often ("high volume" surgeons) have better results than those who perform it less often. The reoperation rates decreased by about 50% in the cohort of patients in whom the surgeon had previous experience of over 200 cases compared to the cohort with a previous experience of less than 200 cases.
Picture gallery
Individual evidence
- ↑ a b c Ioannis Vakalopoulos, Spyridon Kampantais, Leonidas Laskaridis, Vasileios Chachopoulos, Michail Koptsis, Chrysovalantis Toutziaris: New Artificial Urinary Sphincter Devices in the Treatment of Male Iatrogenic Incontinence . In: Advances in Urology . 2012, 2012, pp. 1–6. doi : 10.1155 / 2012/439372 . PMID 22567002 . PMC 3332164 (free full text).
- ↑ a b Thomas Ripert, Jean Pierrevelcin: Comparative study of urodynamic tests after AMS 800 and CSI 375 insertion . In: Urologia Journal . 85, No. 1, February 2018, pp. 15-18. doi : 10.5301 / uj.5000271 . PMID 28967063 .
- ^ A b Ricarda M. Bauer, Christian Gozzi, Wilhelm Hübner, Victor W. Nitti, Giacomo Novara, Andrew Peterson, Jaspreet S. Sandhu, Christian G. Stief: Contemporary Management of Postprostatectomy Incontinence . In: European Urology . 59, No. 6, June 2011, pp. 985-996. doi : 10.1016 / j.eururo.2011.03.020 . PMID 21458914 .
- ↑ a b c d Billy H Cordon, Nirmish Singla, Ajay K Singla: Artificial urinary sphincters for male stress urinary incontinence: current perspectives . In: Medical Devices: Evidence and Research . 2016, No. 9, July 4, 2016, pp. 175–183. doi : 10.2147 / MDER.S93637 . PMID 27445509 . PMC 4938139 (free full text).
- ↑ Eric Chung: Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery . In: Translational Andrology and Urology . 6, No. Supplement 2, July 2017, pp. S112 – S121. doi : 10.21037 / tau . 04.12.2017 . PMID 28791230 . PMC 5522789 (free full text).
- ↑ Frederic EB Foley: An Artificial Sphincter: A New Device and Operation for Control of Enuresis and Urinary Incontinence . In: Journal of Urology . 58, No. 4, October 1947, pp. 250-259. doi : 10.1016 / S0022-5347 (17) 69552-1 . PMID 20266239 .
- ^ F. Brantley Scott, William E. Bradley, Gerald W. Timm: Treatment of Urinary Incontinence By An Implantable Prosthetic Urinary Sphincter . In: Journal of Urology . 112, No. 1, July 1974, pp. 75-80. doi : 10.1016 / S0022-5347 (17) 59647-0 . PMID 4802066 .
- ^ A b Faysal A. Yafi, Mary K. Powers, Jonathan Zurawin, Wayne JG Hellstrom: Contemporary Review of Artificial Urinary Sphincters for Male Stress Urinary Incontinence . In: Sexual Medicine Reviews . 4, No. 2, April 2016, pp. 157–166. doi : 10.1016 / j.sxmr.2015.11.004 . PMID 27872025 .
- ^ A b Oscar A. Suarez, Kurt A. McCammon: The Artificial Urinary Sphincter in the Management of Incontinence . In: Urology . 92, June 2016, pp. 14-19. doi : 10.1016 / j.urology.2016.01.016 . PMID 26845050 .
- ↑ a b c F. B. Scott, WE Bradley, GW Timm: Treatment of urinary incontinence by an implantable prosthetic urinary sphincter . In: The Journal of Urology . 112, No. 1, July 1, 1974, ISSN 0022-5347 , pp. 75-80. doi : 10.1016 / s0022-5347 (17) 59647-0 . PMID 4600662 .
- ^ AMS 800 ™ Urinary Control System For Male Patients: Operating Room Manual . Boston Scientific Corporation, Minnetonka, MN 2017.
- ↑ a b Zephyr Surgical Implants: ARTIFICIAL URINARY SPHINCTER ZSI 375 , Second. Edition, Zephyr Surgical Implants, Geneva, Switzerland November 2019.
- ↑ Ireneusz Ostrowski, Tomasz Golabek, Janusz Ciechan, Emil Śledź, Mikolaj Przydacz, Wojciech Dyś, Mariusz Blewniewski, Burkhard von Heyden, Tobias Pottek, Frank Neugart, Giuseppe Carrieri, Oscar Selvaggio, Francesco Iori, Bob Yang, Steve Fernández Arndez , Christophe Llorens, Waldemar Różanski, Piotr L. Chłosta: Preliminary outcomes of the European multicentre experience with the ZSI 375 artificial urinary sphincter for treatment of stress urinary incontinence in men . In: Central European Journal of Urology . 72, No. 3, 2019, pp. 263-269. doi : 10.5173 / ceju.2019.1920 . PMID 31720028 . PMC 6830485 (free full text).
- ↑ a b Ireneusz Ostrowski, Janusz Ciechan, Emil Sledz, Wojciech Dys, Tomasz Golabek, Piotr L. Chłosta: Four-year follow-up on a ZSI 375 artificial urinary sphincter for male urinary incontinence from one urological center in Poland . In: Central European Journal of Urology . 71, No. 3, 2018, pp. 320-325. doi : 10.5173 / ceju.2018.1704 . PMID 30386654 . PMC 6202622 (free full text).
- ↑ Jaspreet S. Sandhu, Alexandra C. Maschino, Andrew J. Vickers: The Surgical Learning Curve for Artificial Urinary Sphincter Procedures Compared to Typical Surgeon Experience . In: European Urology . 60, No. 6, December 2011, pp. 1285-1290. doi : 10.1016 / j.eururo.2011.05.048 . PMID 21665357 . PMC 3646622 (free full text).
- ↑ Frederic Staerman, Christophe G-Llorens, Priscilla Leon, Yves Leclerc: ZSI 375 artificial urinary sphincter for male urinary incontinence: a preliminary study . In: BJU International . 111, No. 4b, April 2013, pp. E202-E206. doi : 10.1111 / j.1464-410X.2012.11468.x . PMID 22937774 .
- ↑ Alejandro Carvajal Obando, Federico Gavira Gil, Álvaro Gutiérrez Martinez, Luis Fernando Echeverry Molina, Juan Carlos Castaño Botero: EFFICACY OF THE ARTIFICIAL URINARY SPHINCTER ZSI 375 FOR TREATMENT OF POST-RADICAL WITH INTRINCY INTRINCY PATIC: STUDINC . In: European Medical Journal . 2, No. 2, June 1, 2017, pp. 22–26.
- ↑ Ireneusz Ostrowski, Mariusz Blewniewski, Frank Neugart, Burkhard von Heyden, Oscar Selvaggio, Francesco Iori, Steeve Foley, Manuel Fernández Arjona, Alejandro Carvajal Obando, Tobias Pottek: Multicentre experience with ZSI 375 artificial urinary sphincter for the treatment of stress in urinary incontinence men . In: Urologia Journal . 84, No. 3, August 1, 2017, pp. 148–152. doi : 10.5301 / uj.5000246 . PMID 28574143 .
- ↑ a b F.C. Burkhard, JLHR Bosch, F. Cruz, GE Lemack, AK Nambiar, N. Thiruchelvam, A. Tubaro: EAU Guidelines on Urinary Incontinence in Adults . European Association of Urology, Arnhem, The Netherlands 2018, ISBN 978-94-92671-01-1 .
- ↑ Jonathan C. Routh, Douglas A. Husmann: Long-term continence outcomes after immediate repair of pediatric bladder neck lacerations extending into the urethra . In: The Journal of Urology . 178, No. 4S, October 1, 2007, pp. 1816-1818. doi : 10.1016 / j.juro.2007.05.094 . PMID 17707005 .
- ↑ DK Kandpal, SK Rawat, S Kanwar, A Baruha, SK Chowdhary: Single piece artificial urinary sphincter for secondary incontinence following successful repair of post traumatic urethral injury . In: Journal of Indian Association of Pediatric Surgeons . 18, No. 4, 2013, pp. 152–154. doi : 10.4103 / 0971-9261.121120 . PMID 24347870 . PMC 3853858 (free full text).
- ^ A b MAR Islah, Sung Yong Cho, Hwancheol Son: The Current Role of the Artificial Urinary Sphincter in Male and Female Urinary Incontinence . In: The World Journal of Men's Health . 31, No. 1, April 2013, pp. 21-30. doi : 10.5534 / wjmh.2013.31.1.21 . PMID 23658862 . PMC 3640149 (free full text).
- ↑ a b c Jaspreet S. Sandhu, Alexandra C. Maschino, Andrew J. Vickers: The Surgical Learning Curve for Artificial Urinary Sphincter Procedures Compared to Typical Surgeon Experience . In: European Urology . 60, No. 6, 2011, pp. 1285-1290. doi : 10.1016 / j.eururo.2011.05.048 . PMID 21665357 . PMC 3646622 (free full text).
- ↑ artificial sphincter - the operation
- ↑ a b R. Hofmann among others: incontinence and descent surgery of women. Springer Verlag, 2008, ISBN 978-3-540-79937-5 , pp. 219ff., (Online)
- ↑ a b U. Zwergel: Urology specialist examination: In cases, questions and answers. Urban & FischerVerlag, 2008, ISBN 978-3-437-24510-7 , p. 243, (online)
- ↑ a b Urinary Sphincter Replacement (Discharge Care) - What You Need to Know ( en )
- ↑ About Your Artificial Urinary Sphincter | Memorial Sloan Kettering Cancer Center ( en ) Memorial Sloan Kettering Cancer Center.
- ↑ Deepak K Agarwal, Brian J Linder, Daniel S Elliott: Artificial urinary sphincter urethral erosions: Temporal patterns, management, and incidence of preventable erosions . In: Indian Journal of Urology . 0, No. 1, 2016, pp. 26–29. doi : 10.4103 / 0970-1591.195758 . PMID 28197026 . PMC 5264188 (free full text).
- ↑ Daniel S Elliott, David M Barrett, Mohamed Gohma, Timothy B Boone: Does nocturnal deactivation of the artificial urinary sphincter lessen the risk of urethral atrophy? . In: Urology . 57, No. 6, June 2001, pp. 1051-1054. doi : 10.1016 / s0090-4295 (01) 00963-3 . PMID 11377302 .
- ↑ Ireneusz Ostrowski, Mariusz Blewniewski, Frank Neugart, Burkhard von Heyden, Oscar Selvaggio, Francesco Iori, Steeve Foley, Manuel Fernández Arjona, Alejandro Carvajal Obando, Tobias Pottek: Multicentre Experience with ZSI 375 Artificial Urinary Sphincter for the Treatment of Stress Urinary Men . In: Urologia Journal . 84, No. 3, May 29, 2017, pp. 148–152. doi : 10.5301 / uj.5000246 . PMID 28574143 .
- ↑ Christophe Llorens, Tobias Pottek: Urinary Artificial Sphincter ZSI 375 for Treatment of Stress Urinary Incontinence in Men: 5 and 7 Years Follow-Up Report . In: Urologia Journal . 84, No. 4, May 18, 2017, pp. 263–266. doi : 10.5301 / uj.5000243 . PMID 28525665 .
- ↑ a b c d Frank Van der Aa, Marcus J. Drake, George R. Kasyan, Andreas Petrolekas, Jean-Nicolas Cornu: The Artificial Urinary Sphincter After a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non- neurogenic incontinence . In: European Urology . 63, No. 4, April 2013, pp. 681-689. doi : 10.1016 / j.eururo.2012.11.034 . PMID 23219375 .
- ^ A b Drogo K. Montague: Artificial Urinary Sphincter: Long-Term Results and Patient Satisfaction . In: Advances in Urology . 2012, No. Special Issue, 2012. doi : 10.1155 / 2012/835290 . PMID 22536227 . PMC 3318201 (free full text).
- ↑ Eric Chung: A state-of-the-art review on the evolution of urinary sphincter devices for the treatment of post-prostatectomy urinary incontinence: Past, present and future innovations . In: Journal of Medical Engineering & Technology . 38, No. 6, June 17, 2014, pp. 328-332. doi : 10.3109 / 03091902.2014.899400 . PMID 24936961 .
- ↑ Sender Herschorn: The artificial urinary sphincter is the treatment of choice for post-radical prostatectomy incontinence . In: Canadian Urological Association Journal . 2, No. 5, April 17, 2013, pp. 536-9. doi : 10.5489 / cuaj.924 . PMID 18953453 . PMC 2572249 (free full text).
- ↑ Boyd R. Viers, Brian J. Linder, Marcelino E. Rivera, Laureano J. Rangel, Matthew J. Ziegelmann, Daniel S. Elliott: Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence . In: The Journal of Urology . 196, No. 3, 2016, pp. 838-843. doi : 10.1016 / j.juro.2016.03.076 . PMID 26997310 .
- ↑ a b Scott E. Litwiller, Kap B. Kim, Patricia D. Fone, Ralph W. deVere White, Anthony R. Stone: Post-Prostatectomy incontinence and the Artificial Urinary Sphincter: A Long-Term Study of Patient Satisfaction and Criteria for Success . In: Journal of Urology . 156, No. 6, December 1996, pp. 1975-1980. doi : 10.1016 / S0022-5347 (01) 65408-9 . PMID 8911369 .
- ↑ Mahreen Hussain, Tamsin J. Greenwell, Suzie N. Venn, Anthony R. Mundy: The current role of the artificial urinary sphincter for the treatment of urinary incontinence . In: Journal of Urology . 174, No. 2, August 1, 2005, pp. 418-424. doi : 10.1097 / 01.ju.0000165345.11199.98 . PMID 23658862 .
- ^ MAR Islah, Sung Yong Cho, Hwancheol Son: The Current Role of the Artificial Urinary Sphincter in Male and Female Urinary Incontinence . In: The World Journal of Men's Health . 31, No. 2, April 2013, pp. 21-30. doi : 10.5534 / wjmh.2013.31.1.21 . PMID 23658862 .
- ^ Bastian Amend, Patricia Toomey, Karl-Dietrich Sievert: Artificial sphincter . In: Current Opinion in Urology . 23, No. 6, November 2013, pp. 520-527. doi : 10.1097 / 01.MOU.0000434591.02823.d0 . PMID 24080811 .
- ^ Brian J. Linder, Marcelino E. Rivera, Matthew J. Ziegelmann, Daniel S. Elliott: Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic . In: Urology . 86, No. 3, 2015, pp. 602-607. doi : 10.1016 / j.urology.2015.05.029 . PMID 26135815 .
literature
- Christof Börgermann, Albert Kaufmann, Herbert Sperling, Manfred Stöhrer, Herbert Rübben: Therapy of stress incontinence in men: Part 2 of the series incontinence. In: Deutsches Ärzteblatt. July 9, 2010. (aerzteblatt.de)
- Daniel Meyer, Jürg Müller: The artificial urethral sphincter AMS 800 ™ for the treatment of post-prostatectomy incontinence. In: Switzerland Med Forum. 7, 2007, pp. 820-823. (medicalforum.ch, PDF)