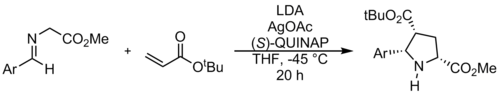Azomethine ylides
Azomethine ylides are nitrogen-containing 1,3- dipoles , which consist of an iminium and a carbanion . They are used in 1,3-dipolar cycloadditions . In this way, heterocycles such as pyrrolidines or pyrrolines can be built up. These reactions are highly stereo- and regioselective and have the potential to generate four contiguous stereocenters. As a result, azomethine ylides are of great use in total synthesis as well as in the manufacture of chiral ligands and drugs. Azomethine ylides can be made from various compounds such as aziridines, imines and iminium ions. Usually the production takes place in situ with a subsequent reaction with a dipolarophile.
structure
The resonance structure shows the 1,3-dipole contributions in which the carbons bound to the nitrogen carry a charge. The most common representation of azomethine ylides shows nitrogen with a positive charge, the negative charge is distributed over the carbon atoms. The actual charge distribution depends on the substituents on the respective atoms. Due to the stabilization, carbons with electron-withdrawing groups have a higher negative partial charge.
Azomethine ylides can (Engl. In three different forms shape ) are present which determine the stereochemistry of the 1,3-dipolar cycloaddition. Ylides can W-shaped ( W-shaped ), U-shaped ( U-shaped ) and S-shaped ( S-shaped to be). The W- and U-shaped ylids, in which the substituents point in the same direction, lead to syn products, while the S-shaped ylides lead to anti products. The position of the substituent R 3 depends on steric and electronic aspects. The stereochemistry of R 1 and R 2 in the product is determined by the dipole. The stereochemistry of R 3 results from the type of dipolarophile. If the dipolarophile is more than monosubstituted and prochiral , four new stereocenters can be established.
Manufacturing
From aziridines
Azomethine ylides can be generated by ring opening of aziridines. In accordance with the Woodward-Hoffmann rules , the thermal 4-electron ring opening takes place in a conrotatory process, while the photochemical reaction takes place in a disrotatory manner.
For asymmetric aziridines, the torque selectivity changes depending on the substituent. Electronegative substituents prefer an outward rotation, while electropositive substituents tend to rotate inward.
In addition to the azomethine ylide, the ring opening of aziridines can also provide another 1,3-dipole if a CN bond is broken instead of the CC bond.
By condensation of aldehydes with amines
One of the simplest methods to generate azomethine ylides is to condense an aldehyde with an amine. If the amine has an electron-withdrawing group on the alpha carbon, such as an ester, it can simply be deprotonated. The disadvantage of this method is that the electron-withdrawing group is also found in the product of the cycloaddition. As an alternative to the ester, a carboxyl group can be used, which can be removed by decarboxylation during the cycloaddition.
From imines and iminium salts
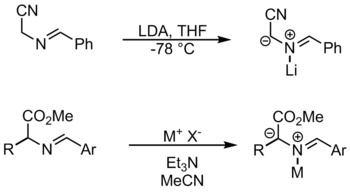
Azomethine ylides can be obtained by direct deprotonation of iminium cations.
Through N metallization
In this method, a metal coordinates on the nitrogen and thus activates the substrate. Among other things, LiBr and AgOAc are used .
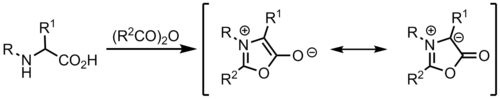
Münchnone
Münchnones are mesoionic heterocycles that can act as azomethine ylides.
1,3-Dipolar Cycloadditions
The 1,3-dipolar cycloaddition with azomethine ylidene is a 6-electron process. According to the Woodward-Hoffmann rules, the addition is suprafacial with regard to both the dipole and the dipolarophile . The reaction is generally considered to be concerted , with both carbon bonds being formed at the same time, but asynchronously. Depending on the nature of the dipole and the dipolarophile, however, diradical or zwitterionic intermediates are also possible. As in the isoelectronic Diels-Alder reaction , the endo product is favored. In this reaction the HOMO of the ylide reacts and the LUMO of the electron-deficient dipolarophile. However, reactions with inactivated π systems are also known, especially when it is an intramolecular cyclization.
In 1,3-dipolar cycloadditions of azomethine ylides, alkenes or alkynes are mostly used as dipolarophiles. This creates pyrrolidines or pyrrolines . Mostly α, β-unsaturated carbonyls are used as dipolarophiles, other functional groups are part of current research.
If the dipole and dipolarophile are part of the same molecule, the intramolecular reaction can lead to polycycles with high complexity. If the dipolarophile is linked to a carbon of the dipole, a fused bicyclic system is created. If the dipolarophile is bound to the nitrogen, a bridged structure is created. Intramolecular variants have the advantage that the regioselectivity is often restricted. Another advantage is that in intramolecular variants the dipolarophile does not have to be electron-poor; variants with electron-rich, alkyl-substituted dipolarophiles are known.
Stereoselectivity of the cycloaddition
While most dipoles lose their stereo information in cycloadditions, azomethine ylides are able to retain their stereochemistry. This is usually achieved by opening an aziridine and then trapping it by a dipolarophile. So the stereochemistry of the Ylides cannot change.
As in other 1,3-dipolar cycloadditions, endo and exo products can be formed. The selectivity can be changed by metal catalysis.
Enantioselective synthesis
Enantioselective cycloadditions of azomethine ylides through the use of chiral catalysts were first described by Allway and Grigg in 1991. This method was further developed by Jørgensen and Zhang. These reactions use metal complexes of Zn, Ag, Cu, Ni and Ca.
Enantiomerically pure spiroindolinones can be synthesized by chiral phosphine catalysis. The method described by Gong et al. leads to an unexpected regiochemistry that is not determined by electronic effects, but by π-stacking with the catalyst.
More reactions
Electrocyclizations
Conjugated azomethine ylides can undergo [1,5] - and [1,7] -electrocyclizations. An example of a [1,7] electrocyclization of a diphenylethenyl substituted azomethine ylide is shown. The conrotatory ring closure is followed by a suprafacial [1,5] hydride shift, which rearomatizes the product. The sterics and geometry of the phenyl ring play a crucial role in this reaction.
The products of this reaction are used in Diels-Alder reactions to modify fullerenes .
In synthesis
Total synthesis of martinellic acid
In this synthesis of martinellic acid , a cycloaddition with an inactivated alkene is used. In this key step, two rings are built, including a pyrrolidine, and two stereocenters.
Total synthesis of spirotryprostatin A
In the synthesis of spirotryprostatin A, an azomethine ylide is obtained by the condensation of an amine with an aldehyde. The ylid reacts with an electron-deficient alkene that is bound to an indolinone. A spiro-fused pyrrolidine and four connected stereocenters are formed.
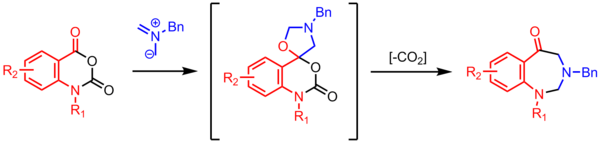
Synthesis of benzodiazepinones
Cyclization of an azomethine ylide with a carbonyl results in a spiro-fused oxazolidine . A 7-membered ring is formed with the elimination of CO 2 .
Individual evidence
- ↑ a b c d e Iain Coldham, Richard Hufton: Intramolecular Dipolar Cycloaddition Reactions of Azomethine Ylides . In: Chemical Reviews . tape 105 , no. 7 , July 2005, ISSN 0009-2665 , p. 2765-2810 , doi : 10.1021 / cr040004c ( acs.org [accessed August 17, 2020]).
- ↑ LM Harwood, RJ Vickers: Azomethine Ylides: Azomethine Ylides . In: Chemistry of Heterocyclic Compounds: A Series Of Monographs . John Wiley & Sons, Inc., New York, USA 2003, ISBN 978-0-471-38726-8 , pp. 169-252 , doi : 10.1002 / 0471221902.ch3 ( wiley.com [accessed August 17, 2020]).
- ↑ Javier Adrio, Juan C. Carretero: Novel dipolarophiles and dipoles in the metal-catalyzed enantioselective 1,3-dipolar cycloaddition of azomethine ylides . In: Chemical Communications . tape 47 , no. 24 , 2011, ISSN 1359-7345 , p. 6784 , doi : 10.1039 / c1cc10779h ( rsc.org [accessed August 17, 2020]).
- ↑ Philippe Dauban, Guillaume Malik: A Masked 1,3-Dipole Revealed from Aziridines . In: Angewandte Chemie International Edition . tape 48 , no. 48 , November 16, 2009, p. 9026-9029 , doi : 10.1002 / anie.200904941 ( wiley.com [accessed August 17, 2020]).
- ↑ Rolf Huisgen, Wolfgang Scheer, Helmut Huber: Stereospecific Conversion of cis-trans Isomeric Aziridines to Open-Chain Azomethine Ylides . In: Journal of the American Chemical Society . 89, No. 7, 1967, pp. 1753-1755. doi : 10.1021 / ja00983a052 .
- ↑ Harold D. Banks: Torquoselectivity Studies in the Generation of Azomethine Ylides from Substituted Aziridines . In: Journal of Organic Chemistry . 75, No. 8, 2010, pp. 2510-2517. doi : 10.1021 / jo902600y . PMID 20329779 .
- ^ Ana L. Cardoso, Teresa MVD Pinho e Melo: Aziridines in Formal [3 + 2] Cycloadditions: Synthesis of Five-Membered Heterocycles . In: European Journal of Organic Chemistry . July 4, 2012, p. n / a – n / a , doi : 10.1002 / ejoc.201200406 ( wiley.com [accessed August 17, 2020]).
- ↑ Edward Huie: Intramolecular [3 + 2] cycloaddition routes to carbon-bridged dibenzocycloheptanes and dibenzazepines . In: Journal of Organic Chemistry . 48, No. 18, 1983, pp. 2994-2997. doi : 10.1021 / jo00166a011 .
- ^ Albert Padwa, Henry L. Gingrich, Richard Lim: Regiochemistry of intramolecular munchnone cycloadditions: preparative and mechanistic implications . In: The Journal of Organic Chemistry . tape 47 , no. June 12 , 1982, ISSN 0022-3263 , pp. 2447–2456 , doi : 10.1021 / jo00133a041 ( acs.org [accessed August 17, 2020]).
- ↑ Kendall N. Houk, Javier Gonzalez, Yi Li: Pericyclic Reaction Transition States: Passions and Punctilios, 1935-1995 . In: Accounts of Chemical Research . tape 28 , no. 2 , February 1995, ISSN 0001-4842 , p. 81-90 , doi : 10.1021 / ar00050a004 ( acs.org [accessed August 18, 2020]).
- ↑ Brad R. Henke, Andrew J. Kouklis, Clayton H. Heathcock: Intramolecular 1,3-dipolar cycloaddition of stabilized azomethine ylides to unactivated dipolarophiles . In: The Journal of Organic Chemistry . tape 57 , no. December 26 , 1992, ISSN 0022-3263 , pp. 7056-7066 , doi : 10.1021 / jo00052a015 ( acs.org [accessed August 18, 2020]).
- ↑ Chuo Chen, Xiaodong Li, Stuart L. Schreiber: Catalytic Asymmetric [3 + 2] Cycloaddition of Azomethine Ylides. Development of a Versatile Stepwise, Three-Component Reaction for Diversity-Oriented Synthesis . In: Journal of the American Chemical Society . tape 125 , no. 34 , August 2003, ISSN 0002-7863 , p. 10174-10175 , doi : 10.1021 / ja036558z ( acs.org [accessed August 18, 2020]).
- ↑ Javier Adrio, Juan C. Carretero: Novel dipolarophiles and dipoles in the metal-catalyzed enantioselective 1,3-dipolar cycloaddition of azomethine ylides . In: Chemical Communications . tape 47 , no. 24 , 2011, ISSN 1359-7345 , p. 6784 , doi : 10.1039 / c1cc10779h ( rsc.org [accessed August 18, 2020]).
- ↑ Wenzhong Gao, Xumu Zhang, Malati Raghunath: Cu (I) -Catalyzed Highly Exo-selective and Enantioselective [3 + 2] Cycloaddition of Azomethine Ylides with Acrylates . In: Organic Letters . tape 7 , no. September 19 , 2005, ISSN 1523-7060 , p. 4241-4244 , doi : 10.1021 / ol0516925 ( acs.org [accessed August 18, 2020]).
- ↑ Ichiro Oura, Kenta Shimizu, Kenichi Ogata, Shin-ichi Fukuzawa: Highly Endo -Selective and Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Ylide with α-Enones Catalyzed by a Silver (I) / ThioClickFerrophos Complex . In: Organic Letters . tape 12 , no. 8 , April 16, 2010, ISSN 1523-7060 , p. 1752–1755 , doi : 10.1021 / ol100336q ( acs.org [accessed August 18, 2020]).
- ↑ Philip Allway, Ronald Grigg: Chiral Co (II) and Mn (II) catalysts for the 1,3-dipolar cycloaddition reactions of azomethine ylides derived from arylidenes imine of glycine . In: Tetrahedron Letters . tape 32 , no. 41 , October 1991, p. 5817–5820 , doi : 10.1016 / S0040-4039 (00) 93563-9 ( elsevier.com [accessed August 18, 2020]).
- ↑ Xiao-Hua Chen, Qiang Wei, Shi-Wei Luo, Han Xiao, Liu-Zhu Gong: Organocatalytic Synthesis of Spiro [pyrrolidine-3,3'-oxindoles] with High Enantiopurity and Structural Diversity . In: Journal of the American Chemical Society . tape 131 , no. 38 , September 30, 2009, ISSN 0002-7863 , p. 13819–13825 , doi : 10.1021 / ja905302f ( acs.org [accessed August 18, 2020]).
- ↑ NA Nedolya, BA Trofimov: [1,7] Electrocyclization reactions in the synthesis of azepine derivatives . In: Chemistry of Heterocyclic Compounds . tape 49 , no. 1 , April 2013, ISSN 0009-3122 , p. 152–176 , doi : 10.1007 / s10593-013-1236-y ( springer.com [accessed August 18, 2020]).
- ↑ Judit Tóth, András Dancsó, Gábor Blaskó, László Tőke, Paul W. Groundwater: 1.7 Electrocyclization reactions of stabilized α, β: γ, δ- Unsaturated azomethine ylides . In: Tetrahedron . tape 62 , no. June 24 , 2006, pp. 5725–5735 , doi : 10.1016 / j.tet.2006.03.088 ( elsevier.com [accessed August 18, 2020]).
- ↑ Jean-François Eckert, Cyril Bourgogne, Jean-François Kidney Garden: An unexpected Diels – Alder reaction on the fullerene core rather than an expected 1,3-dipolar cycloaddition . In: Chemical Communications . No. 7 , March 21, 2002, p. 712–713 , doi : 10.1039 / b201122k ( rsc.org [accessed August 18, 2020]).
- ↑ Barry B. Snider, Yong Ahn, Sean M. O'Hare: Total Synthesis of (±) -Martinellic Acid . In: Organic Letters . tape 3 , no. December 26 , 2001, ISSN 1523-7060 , p. 4217–4220 , doi : 10.1021 / ol016884o ( acs.org [accessed August 18, 2020]).
- ↑ Tomoyuki Onishi, Paul R. Sebahar, Robert M. Williams: Concise, Asymmetric Total Synthesis of spirotryprostatin A . In: Organic Letters . tape 5 , no. August 17 , 2003, ISSN 1523-7060 , p. 3135-3137 , doi : 10.1021 / ol0351910 ( acs.org [accessed August 18, 2020]).
- ↑ Nadia Spiccia, Jose Basutto, Pawel Jokisz, Leon S.-M. Wong, Adam G. Meyer: 1,3-Dipolar Cycloaddition - Decarboxylation Reactions of an Azomethine Ylide with Isatoic Anhydrides: Formation of Novel Benzodiazepinones . In: Organic Letters . tape 13 , no. 3 , February 4, 2011, ISSN 1523-7060 , p. 486-489 , doi : 10.1021 / ol102824k ( acs.org [accessed August 18, 2020]).