Benzotrifuroxane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
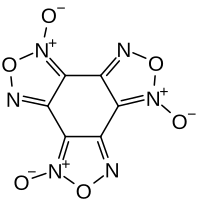
|
||||||||||
| General | ||||||||||
| Surname | Benzotrifuroxane | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 6 N 6 O 6 | |||||||||
| Brief description |
colorless crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 252.103 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.87 g cm −3 |
|||||||||
| Melting point |
195 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Benzotrifuroxane is a heterocyclic organic compound that can be assigned to the group of 1,2,5- oxadiazoles . It is formally derived from the nonexistent hexanitrosobenzene. The high-energy compound is an explosive .
history
The compound was first synthesized in 1924 by O. Turek as hexanitrosobenzene . In addition to the hexanitroso structure, symmetrical polycyclic structures could also be formulated.
Investigations using IR and Raman spectroscopy as well as single crystal X-ray diffraction showed that these structures are not real and that the compound is present as a tetracyclic benzotrifuroxane structure.
Extraction and presentation
Benzotrifuroxane can be obtained by the thermal degradation of 1,3,5-triazido-2,4,6-trinitrobenzene .
Another synthesis can be carried out by reacting 5,7-dichloro-4,6-dinitronbenzofuroxan with sodium azide .
properties
Physical Properties
Benzotrifuroxane is a crystalline solid that melts at 195 ° C. The compound crystallizes in an orthorhombic crystal lattice with the space group Pna2 1 . The molar enthalpy of formation is 606 kJ mol −1 , the enthalpy of combustion −2967 kJ mol −1 .
Chemical properties
Benzotrifuroxane can break down explosively. The heat of the explosion is 5903 kJ kg −1 , the detonation speed 8.61 km s −1 . The connection is sensitive to impact .
Benzotrifuroxane forms stable complexes with aromatic hydrocarbons such as naphthalene , 1-phenylnaphthalene , 2-phenylnaphthalene and tetrahydronaphthalene . A recrystallization in benzene yields a 1: 1 complex, wherein the benzene only at 100 ° C in the vacuum can be removed.
use
In combination with TNT , the compound can be used to produce nanodiamonds by means of detonation shock waves .
Individual evidence
- ↑ a b A. S. Bailey, JR Case: 4: 6-dinitrobenzofuroxan, nitrobenzodifuroxan and benzotrifuroxan: A new series of complex-forming reagents for aromatic hydrocarbons . In: Tetrahedron . tape 3 , no. 2 , 1958, p. 113-131 , doi : 10.1016 / 0040-4020 (58) 80003-4 .
- ↑ a b c H. H. Cady, AC Larson, DT Cromer: The Crystal Structure of Benzotrifuroxan (Hexanitrosobenzene) . In: Acta Crystallographica . tape 20 , no. 3 , March 1, 1966, p. 336-341 , doi : 10.1107 / S0365110X6600080X .
- ↑ a b J. CA Boeyens, FH Herbstein: Molecular Compounds and Complexes. II. Exploratory Crystallographic Study of Some Donor-Acceptor Molecular Compounds 1 . In: The Journal of Physical Chemistry . tape 69 , no. 7 , July 1, 1965, p. 2153-2159 , doi : 10.1021 / j100891a003 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b O. Turek: Le 2,4,6-trinitro-1,3,5-triazido-benzene, new explosif d'amorcage . In: Chimie et industrie . tape 26 , 1931, p. 781-794 .
- ↑ a b O. Turek: 1,3,5-Triazido-2,4,6-trinitrobenzen, nova inicialna vybusina . In: Chemicky obzor . No. 7 , 1932, pp. 76-79; 97-104 .
- ^ Neville Bacon, AJ Boulton, AR Katritzky: Structure of “hexanitrosobenzene” from vibrational spectroscopy . In: Transactions of the Faraday Society . tape 63 , no. 0 , January 1, 1967, p. 833-835 , doi : 10.1039 / TF9676300833 .
- ↑ EA Chugunova, RE Timasheva, EM Gibadullina, AR Burilov, R. Goumont: First Synthesis of Benzotrifuroxan at Low Temperature: Unexpected Behavior of 5,7-Dichloro-4,6-dinitrobenzo-furoxan with Sodium Azide . In: Propellants, Explosives, Pyrotechnics . tape 37 , no. 4 , July 20, 2012, p. 390–392 , doi : 10.1002 / prep.201200080 .
- ↑ EN Maslen: A phase refinement of the crystal structure of benzotrifuroxan . In: Acta Crystallographica Section B, Structural Crystallography and Crystal Chemistry . tape 24 , no. 9 , September 1, 1968, pp. 1170-1172 , doi : 10.1107 / S0567740868003912 .
- ^ Prince E. Rouse: Enthalpies of formation and calculated detonation properties of some thermally stable explosives . In: Journal of Chemical & Engineering Data . tape 21 , no. 1 , January 1, 1976, p. 16-20 , doi : 10.1021 / je60068a026 .
- ↑ Betsy M. Rice, Jennifer Hare: Predicting heats of detonation using quantum mechanical calculations . In: Thermochimica Acta . tape 384 , no. 1-2 , February 25, 2002, pp. 377-391 , doi : 10.1016 / S0040-6031 (01) 00796-1 .
- ↑ H. Muthurajan, R. Sivabalan, MB Talawar, SN Asthana: Computer simulation for prediction of performance and thermodynamic parameters of high energy materials . In: Journal of Hazardous Materials . tape 112 , no. 1–2 , August 9, 2004, pp. 17-33 , doi : 10.1016 / j.jhazmat.2004.04.012 .
- ↑ VI Pepekin, BL Korsunskii, AA Denisaev: initiation of solid explosives by mechanical impact . In: Combustion, Explosion, and Shock Waves . tape 44 , no. 5 , September 1, 2008, p. 586-590 , doi : 10.1007 / s10573-008-0089-7 .
- ^ NV Kozyrev: Using the tracer method to study detonation processes . In: Combustion, Explosion, and Shock Waves . tape 44 , no. 6 , November 1, 2008, p. 698-703 , doi : 10.1007 / s10573-008-0105-y .